Oral delivery of macromolecules
A macromolecular, selected technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of non-transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
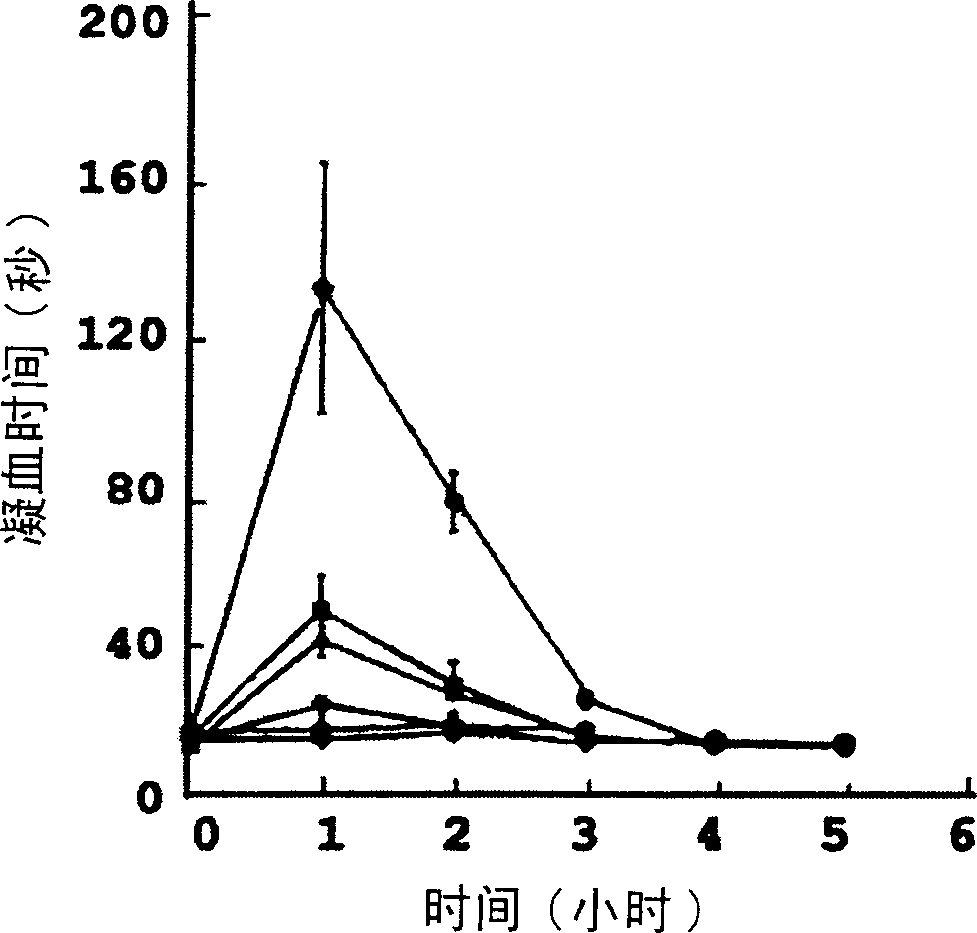
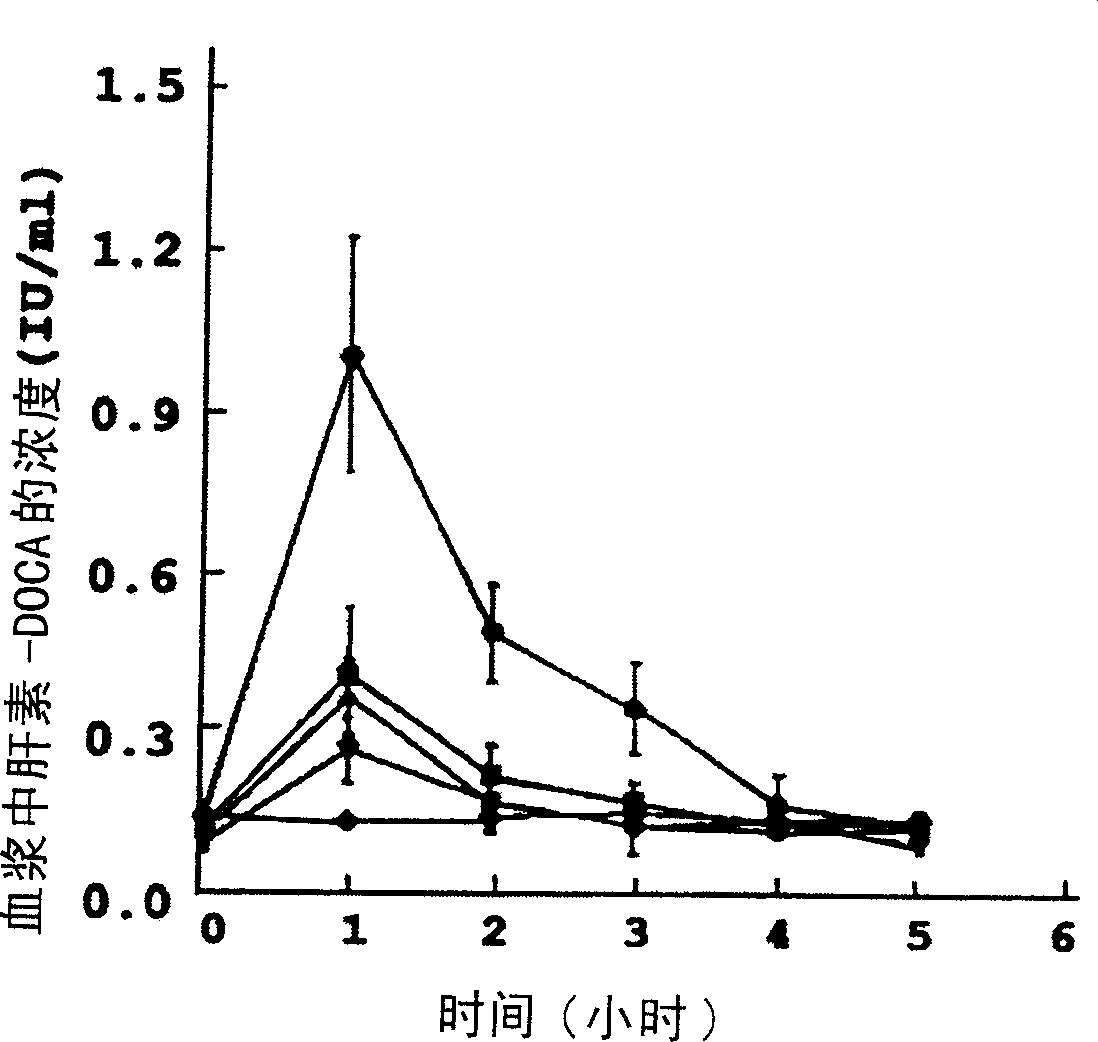
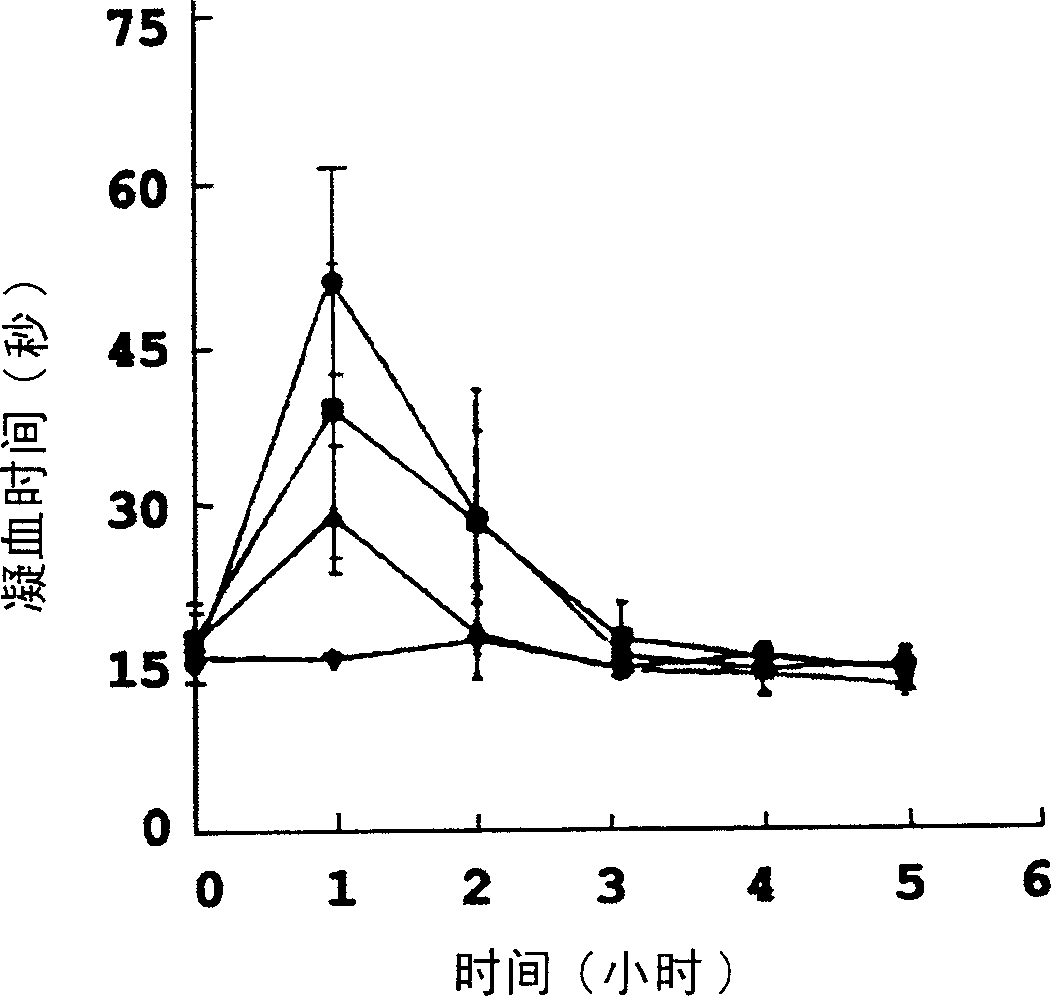
Image
Examples
Embodiment 1
[0076] Synthesis of Heparin-DOCA Conjugate
[0077]A dimethylformamide (DMF) solution of 5 ml of N-hydroxysuccinimide (HOSu, 92 mg / 5 ml) was mixed with a DMF solution of 5 ml of dicyclohexylcarbodiimide (DCC) (165 mg / 5 ml), Then 5ml of DOCA (196mg / 5ml) in DMF was added. The molar ratio of DOCA, HOSu and DCC is 1:1.6:1.6. The concentrations of HOSu and DCC were slightly higher than that of DOCA to fully activate DOCA. The resulting solution was reacted at room temperature under vacuum for 5 hours, and then dicyclohexylurea (DCU), a by-product precipitated during the reaction, was removed. Unreacted DCC was removed by dropwise addition of distilled water and filtration. The remaining HOSu was then removed by adding 15 ml of distilled water. Activated DOCA was precipitated and then lyophilized. The activated DOCA was then dissolved in DMF and reacted with heparin at room temperature for 4 hours. The amount of heparin used in these reactions was 40-400 mg. After the reactio...
Embodiment 2
[0081] Preparation of heparin-cholesterol conjugates
[0082] The hydroxyl groups of cholesterol are activated by reaction with chloroacetic acid to generate free carboxyl groups. This modified cholesterol was then reacted with a solution of HOSu and DCC in 10 ml of DMF following the procedure of Example 1. The molar ratio of cholesterol, HOSu and DCC was 1:1.6:1.6, and the reaction was carried out at room temperature for 5 hours. To remove unreacted DCC and HOSu, water was added and the solution was filtered through a 0.45 μm membrane. Next, the activated cholesterol was reacted with the heparin solution for 4 hours. Two products are obtained from this reaction: a water-soluble product and a water-insoluble product. These products were worked up according to the procedure in Example 1 above.
Embodiment 3
[0084] Synthesis of heparin-alkanoic acid conjugates
[0085] The procedure of Example 1 was followed to couple lauric and palmitic acids to heparin. The carboxyl group of the alkanoic acid is coupled with the amine group of heparin to form an amide bond. Coupling reagents include HOSu and DCC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com