Iron chelator-containing prothrombin time reagent
A technology of prothrombin time and iron chelating agent, which is applied in the field of coagulation diagnosis and can solve problems such as poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
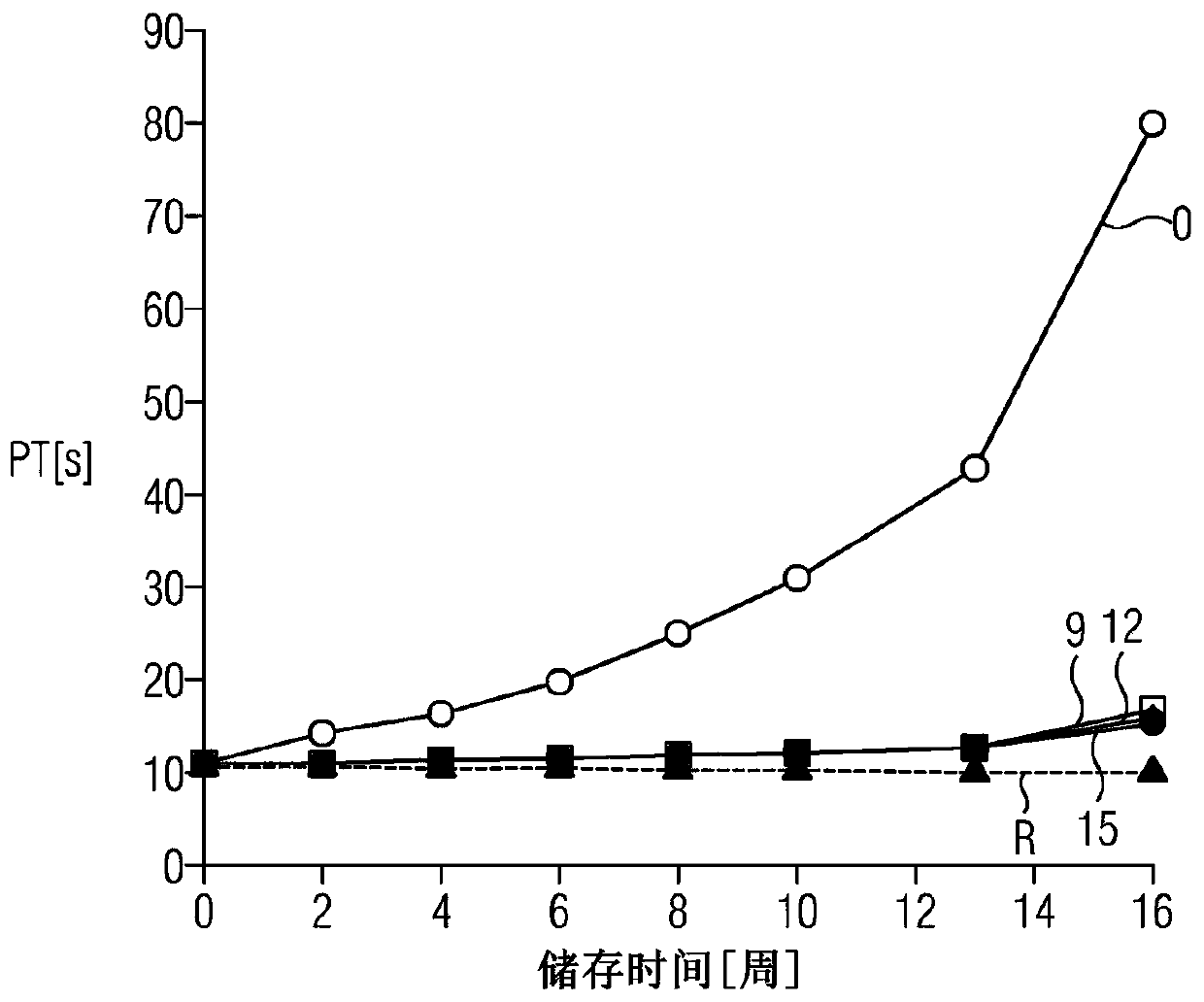
[0037] Example 1: Determination of the Stability of Liquid Prothrombin Time Reagent Stabilized by Desferrioxamine
[0038] at time point t 0 , deferoxamine (deferoxamine mesylate, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was added in different batches to liquid coagulation containing recombinant human tissue factor protein, synthetic phospholipids and calcium ions at the following final concentrations In the zymogen time reagent:
[0039] 1.0 mM deferoxamine,
[0040] 2.0.0137mM (9mg / L) deferoxamine,
[0041] 3.0.0183mM (12mg / L) deferoxamine,
[0042] 4.0.0228mM (15mg / L) deferoxamine,
[0043] 5.0.114mM (75mg / L) deferoxamine,
[0044] 6. 0.228mM (150mg / L) deferoxamine.
[0045] exist Different reagents were used in the automated prothrombin time test (PT test) in the CA-1500 analyzer (Sysmex Corp., Kobe, Japan). Use the control plasma specified below as a sample:
[0046] · Clotting Control Level 1 (Ci-Trol 1) control plasma; normal control used to determine ...
Embodiment 2
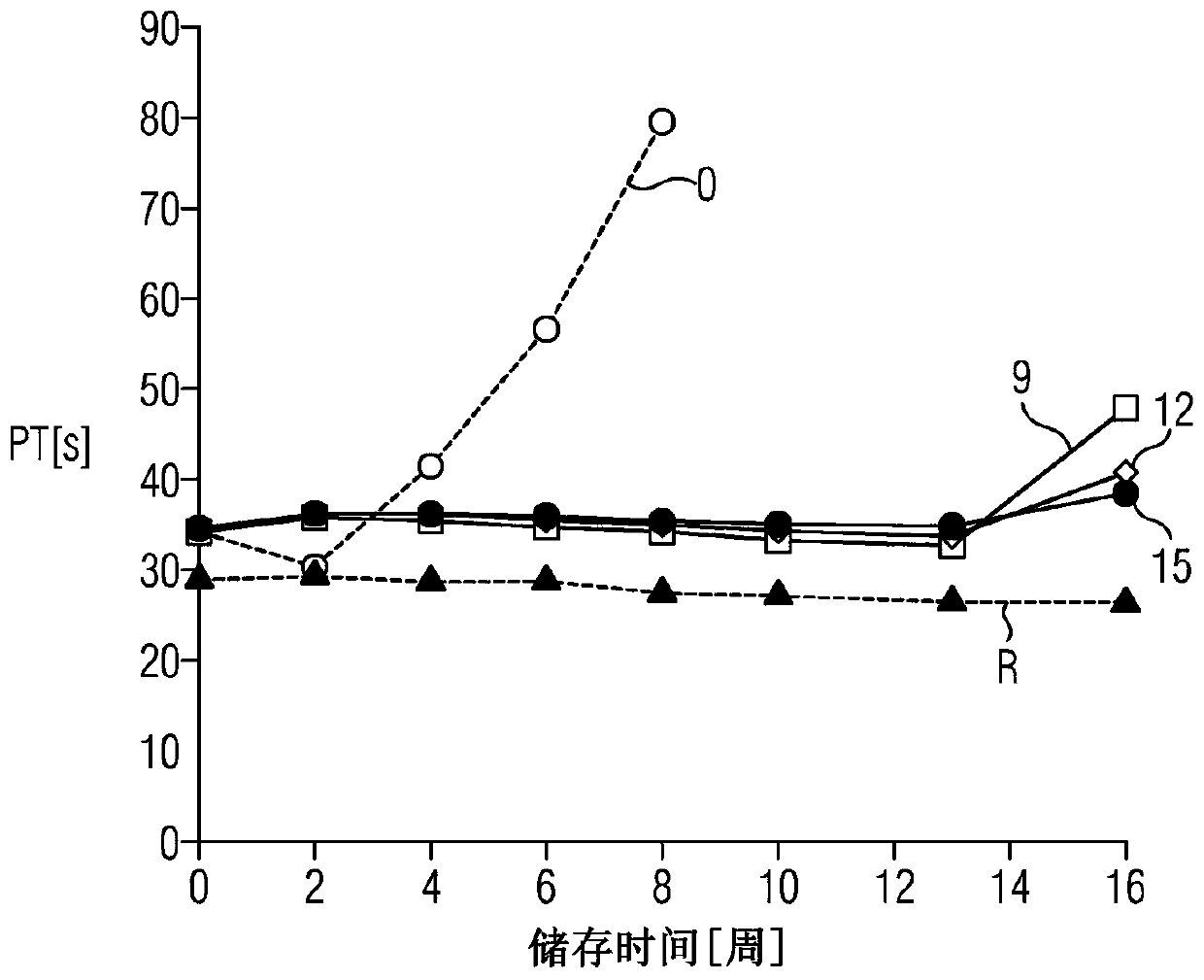
[0052] Example 2: Determination of the stability of liquid prothrombin time reagents stabilized with different iron chelators
[0053] at time point t 0 In different batches, the following metal ion chelating agents were added to the liquid prothrombin time reagent containing recombinant human tissue factor protein, synthetic phospholipids and calcium ions at 0.0228mol / L:
[0054] i. Fluorescent siderophores (Pyoverdines, Sigma-Aldrich Chemie GmbH, Steinheim, Germany);
[0055] ii. Iron pigment (free iron iron pigment from Ustilago sphaerogena, Sigma-Aldrich Chemie GmbH, Steinheim, Germany);
[0056]iii. deferoxamine (deferoxamine mesylate, Sigma-Aldrich Chemie GmbH, Steinheim, Germany); deferoxamine (deferoxamine mesylate, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) );
[0057] iv. Deerasirox (Combi-Blocks, Inc., San Diego, USA);
[0058] v.DTPA (diethylenetriaminepentaacetic acid);
[0059] vi.EDTA (ethylenediaminetetraacetic acid);
[0060] vii. BAPTA (1,2-bis(2-am...
Embodiment 3
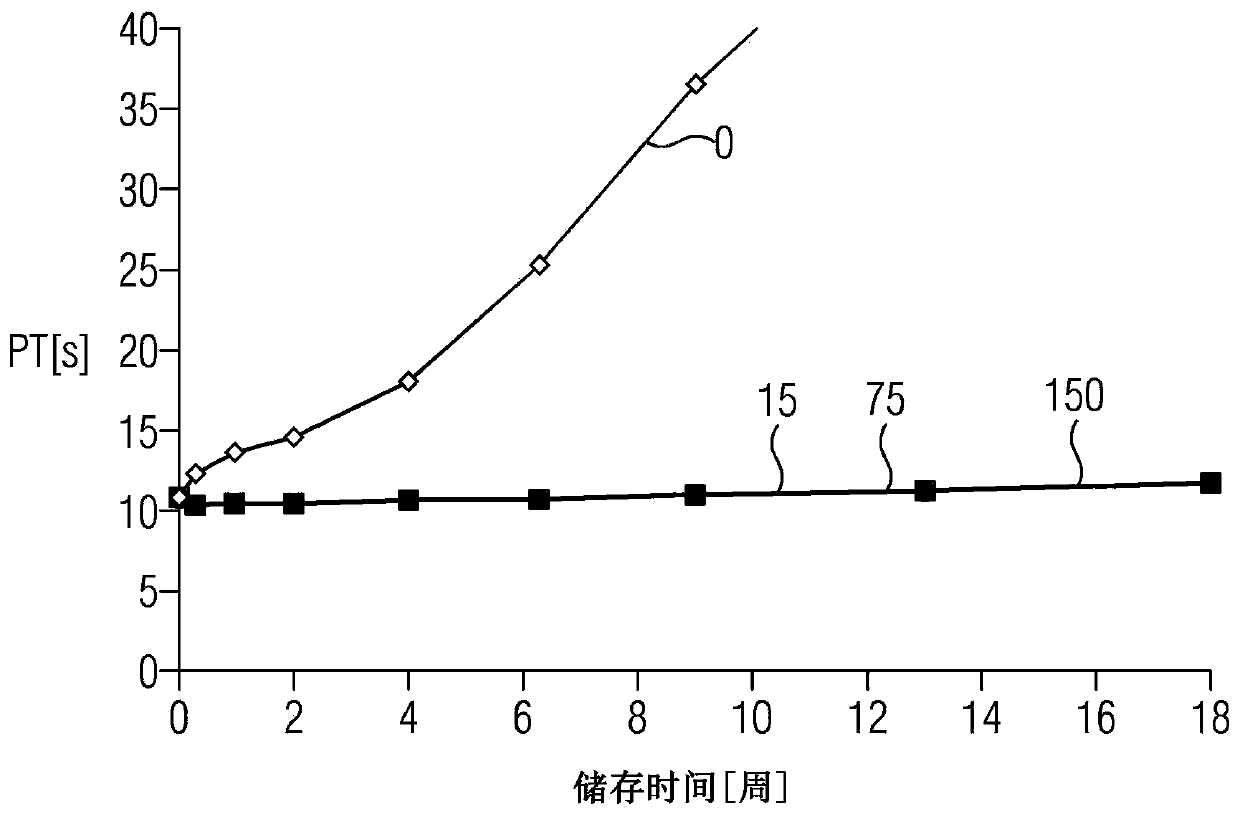
[0069] Example 3: Determination of the presence of higher Fe 3+ Stability of liquid prothrombin time reagents stabilized with different iron chelators at ionic concentrations
[0070] at time point t 0 , the FeCl of 0.00308mM (0.5mg / L) 3 Added to the liquid prothrombin time reagent containing recombinant human tissue factor protein, synthetic phospholipids and calcium ions, and then added 0.0228mM of the metal ion chelating agent mentioned in Example 2 in different batches.
[0071] As described in Example 1, in Different reagents were used in the automated prothrombin time test (PT test) in the CA-1500 analyzer (Sysmex Corp., Kobe, Japan). Quality control plasma Ci-Trol 1 and Ci-Trol 2 described in detail in Example 1 were used as samples.
[0072] In order to determine the presence of higher Fe 3+ Long-term stability of the different reagents at ion concentration, the reagents were stored in liquid state at +37°C in stoppered glass vials over a period of 6 weeks. Reag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com