Maintenance of Platelet Inhibition During Antiplatelet Therapy
a technology of antiplatelet therapy and platelet activity, which is applied in the field of platelet inhibition, can solve the problems of vascular occlusion and ischemic damage, dual antiplatelet therapy, increased bleeding complications, and increased blood transfusion rates of patients receiving dual antiplatelet therapy, so as to maintain or reduce platelet activity, the effect of not increasing the risk of bleeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
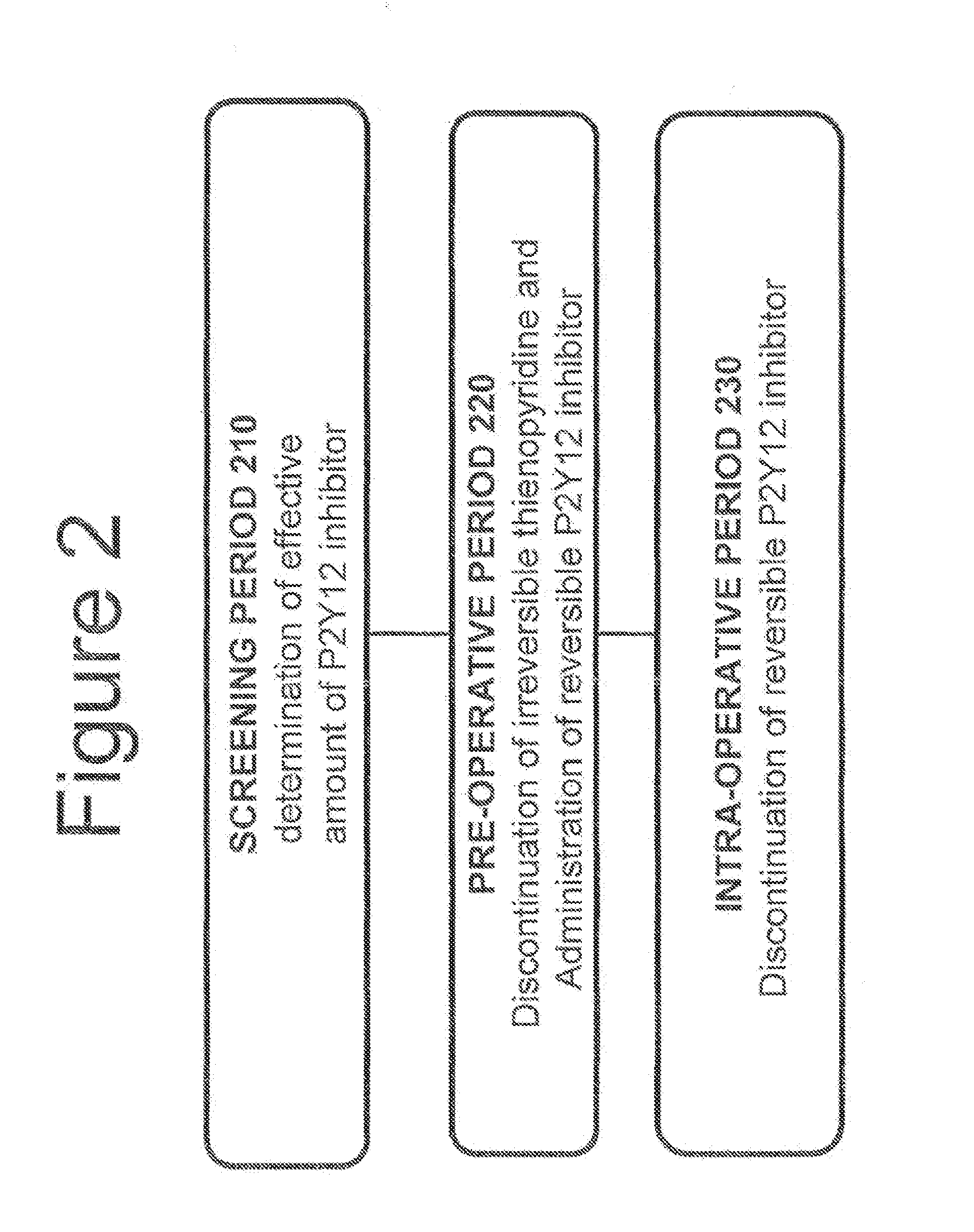
[0093]Without limitations, FIG. 2 provides a brief summary as to how the methods described in the present invention may be used in a patient in need thereof. It should be understood that the method of the present invention is not limited to the procedure described in FIG. 2.
[0094]FIG. 2, shows a screening period 210 used for determining the dosage necessary for achieving platelet inhibition greater than a pre-determined level, for example, of approximately 60%. A pre-operative period 220 of up to approximately 7 days prior to surgery can be used for administration of a reversible, short-acting P2Y12 inhibitor. An intra-operative period 230 lasting from the discontinuation of the reversible, short-acting P2Y12 inhibitor to the end of surgery can be used.
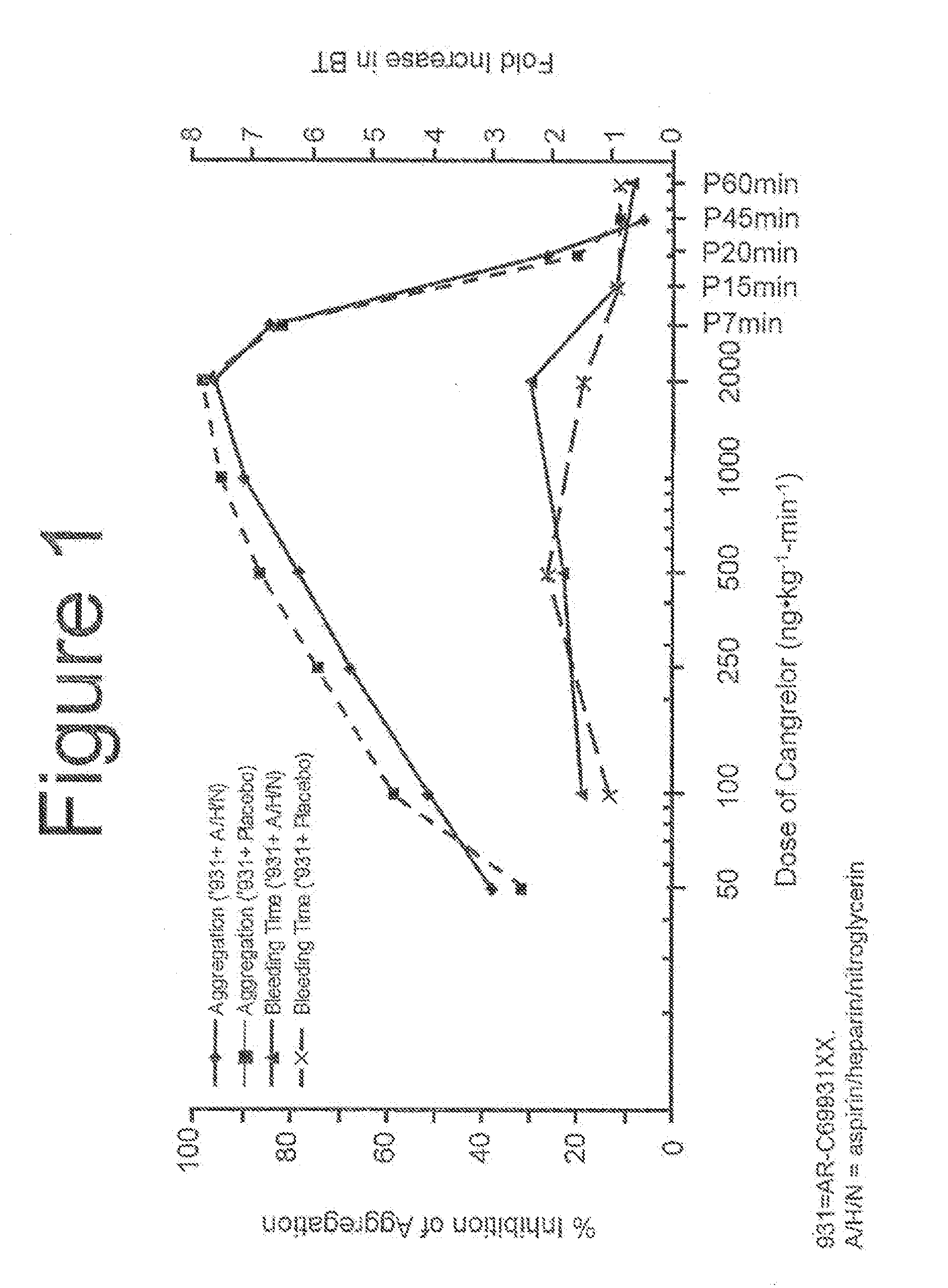
[0095]During the screening period 210, the dosage of a reversible, short-acting P2Y12 inhibitor, necessary to achieve platelet inhibition greater than approximately 60% can be determined. Other suitable levels of percent inhibition ar...
example 2
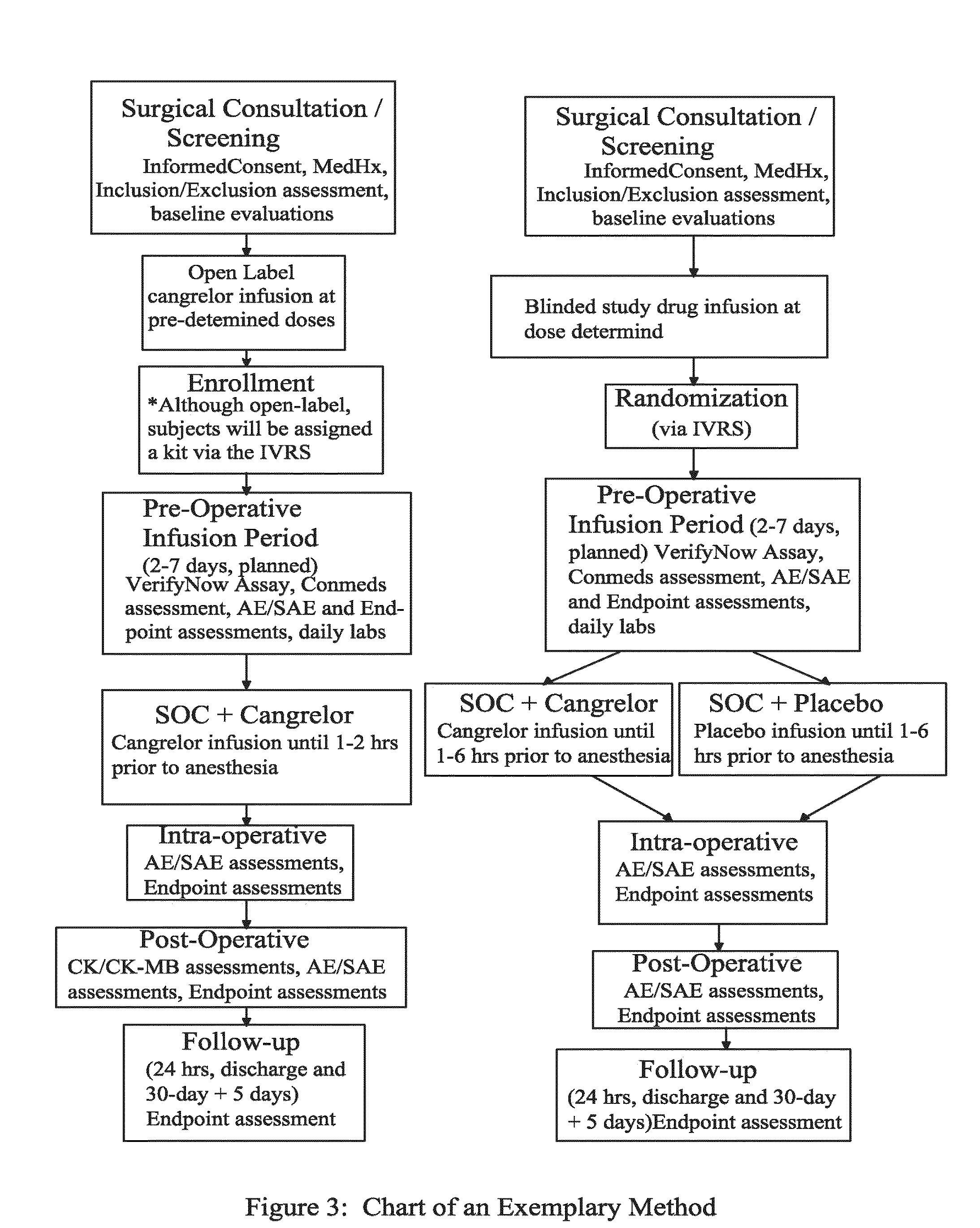
[0101]FIG. 3 describes a non-limiting exemplary method for maintaining or reducing platelet activity in patients who were previously treated with thienopyridine prior to undergoing an invasive procedure such as CABG.
[0102]In FIG. 3, a total of 207 patients were studied. Patients were included in this study if they met all of the following criteria: (1) Must be at least 18 years of age; (2) Anticipate non-emergent coronary artery bypass graft (CABG) surgery, either “on-pump” or “off-pump,” no sooner than 48 hours from randomization but no longer than 7 days from randomization, with patient to remain hospitalized until planned CABG; and (3) Have received a thienopyridine (at least 75 mg of clopidogrel, 500 mg ticlopidine, or 10 mg prasugrel) within 72 hours prior to enrollment in the study for either the treatment of an acute coronary syndrome, regardless of time from ACS, and / or as long-term preventative therapy following drug-eluting or bare metal stent treatment.
[0103]Patients were...
example 3
[0121]In another example and in accordance with one embodiment of the present invention, the administration of the at least one reversible, short-acting P2Y12 inhibitor occurs during an invasive procedure being performed on the subject. In this manner, it is contemplated that the administration of the inhibitor would occur intravenously as the subject cannot take the therapy orally.
PUM
| Property | Measurement | Unit |
|---|---|---|
| fill volume | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com