Preparation method of polypeptide solid-phase synthesis bivalirudin crude product
A peptide solid-phase synthesis, bifaludin technology, applied in the field of peptide solid-phase synthesis, can solve the problems of cumbersome operation, high cost, limited product purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
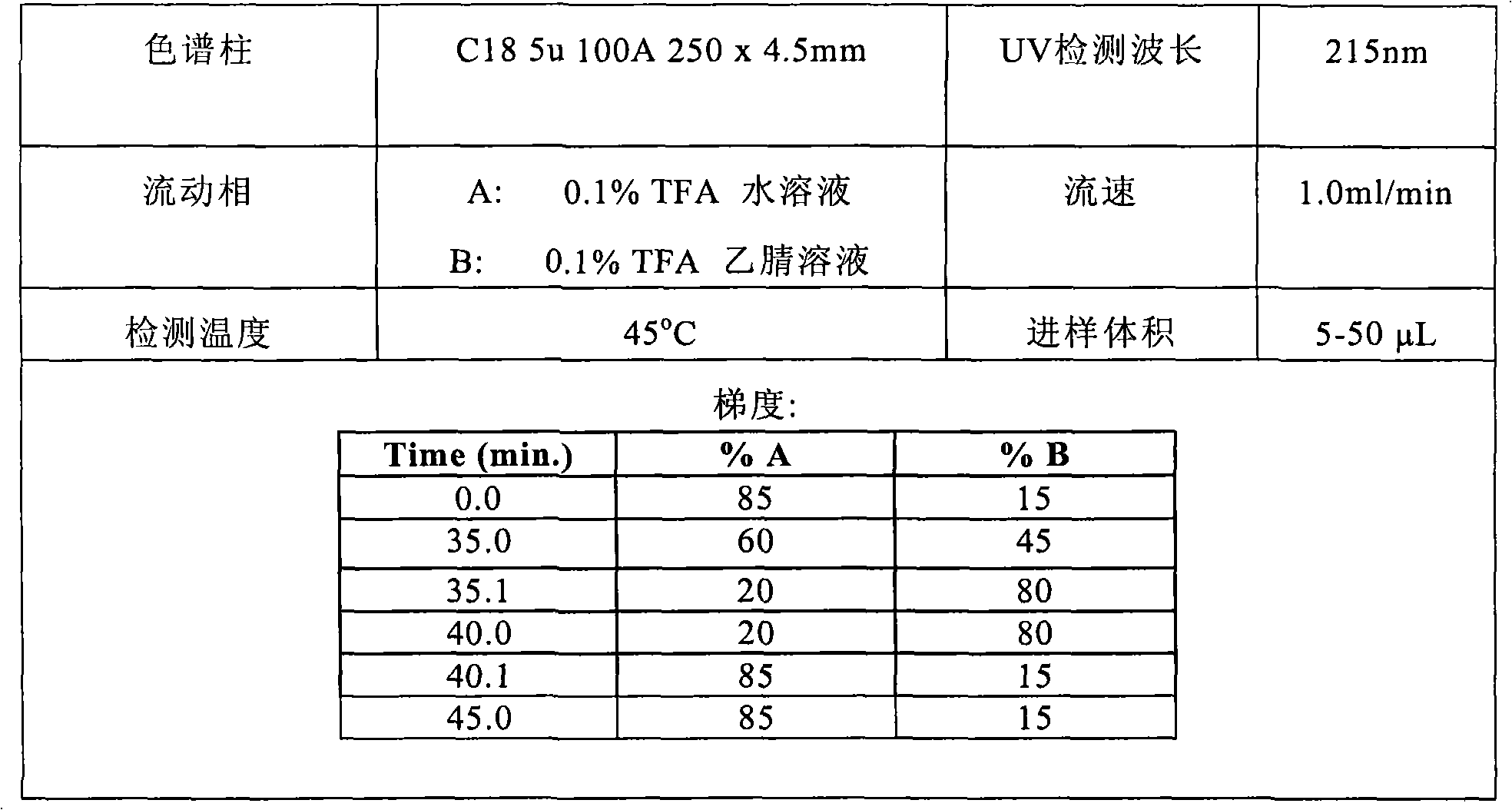
Image
Examples
preparation example Construction
[0047] In an example of the present invention, the preparation method of polypeptide solid-phase synthesis bivalirudin of the present invention comprises the following steps:
[0048] In the first step, Fmoc-leucine (Fmoc-Leu) is bonded to the resin. The resin used can be well known in the art, preferably Wang resin, more preferably the substitution rate of Wang resin is 1.0-1.4 mmol / g.
[0049] The second step is to use the deprotecting agent of the present invention to remove the Fmoc group, that is, to wash the Fmoc-Leu-resin obtained in the first step with the deprotecting agent of the present invention to remove the Fmoc group.
[0050] The third step is to condense the Fmoc-amino acid and the amino acid on the resin. In a preferred example of the present invention, the amino acid is condensed one by one from the C-terminal to the N-terminal according to the polypeptide shown in formula I until it is bonded to the resin. There is a polypeptide as shown in formula I.
[...
Embodiment 1
[0072] Preparation of Bifaludine 1
[0073] step one:
[0074] Fmoc-leucine bonding step: 2.0 equivalent mol Fmoc-leucine, activated by 2,6-dichlorobenzoyl chloride and pyridine and Wang resin (substitution rate 1.2-1.4mmol / g) in N,N-di reaction in methylformamide solution.
[0075] Step two:
[0076] Fmoc removal procedure: Fmoc group was removed with 15% piperidine / 5% DBU in DMF for 30 minutes. Then wash the resin 1 time with DMF; wash the resin 3 times with methanol; wash the resin 3 times with DMF.
[0077] Fmoc-amino acid (the last amino acid condensation of Boc-D-Phe-OH) condensation step: 2.0-3.0 times of resin equivalent of Fmoc-amino acid, 1.5 times of resin equivalent of HOBt dissolved in 3mL / g resin amount of DMF and added to the resin , and then add 3.0 times of resin equivalent DIC. After reacting for 90 minutes, monitor with ninhydrin chromogenic method (Kaiser), control the temperature to 10°C, and then dilute the above solution to a volume of 4mL / g resin wi...
Embodiment 2
[0087] Preparation of Bifaludin 2
[0088] Fmoc-leucine bonding step: 3.0 equivalent mol Fmoc-leucine is activated by 2,6-dichlorobenzoyl chloride and pyridine and Wang resin (substitution rate 1.0-1.2mmol / g) in N,N-dimethyl reaction in the formamide solution. Unreacted groups on the resin were blocked with benzoyl chloride / triethylamine.
[0089] Step two:
[0090]Fmoc removal step: 10% piperidine / 7% DBU / 3% HOOBT DMF solution of 5 times the volume of the resin bed was used for 30 minutes to remove the Fmoc group. Then wash the resin with 5 times the resin bed volume of DMF for 1 time; wash the resin with 5 times the resin bed volume of methanol for 3 times; wash the resin with 5 times the resin bed volume of DMF for 3 times.
[0091] Fmoc-amino acid (the last amino acid condensation of Boc-D-Phe-OH) condensation step: 1.5-2.0 times of resin equivalent of Fmoc-amino acid, 3.0 times of resin equivalent of HOBt dissolved in 5mL / g resin amount of DMF and added to the resin , ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com