Preparation method of bivalirudin
A bivalirudin and molar ratio technology, applied in the field of peptide synthesis, can solve the problems of many process control points, complex steps of bivalirudin, unstable process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
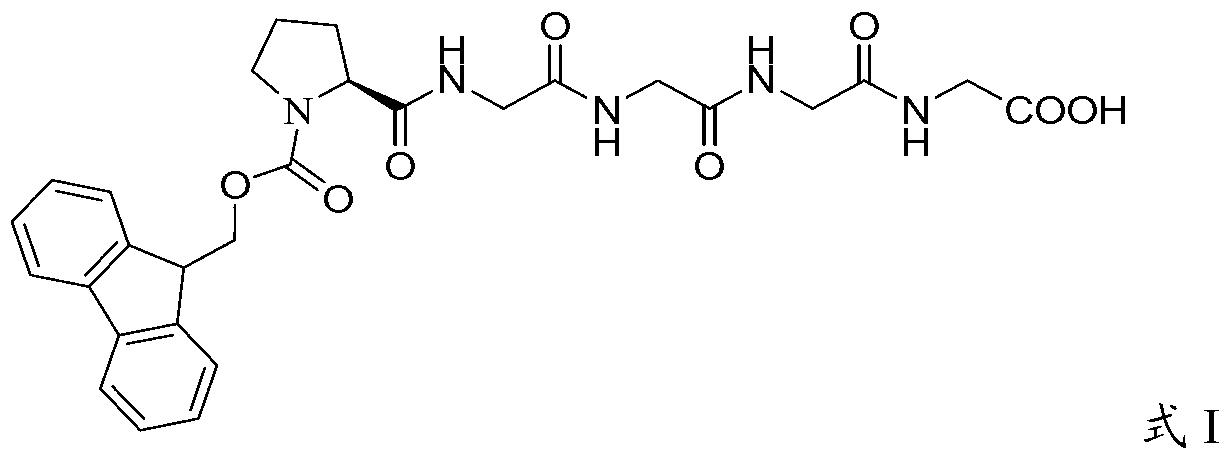
[0061] Embodiment 1, the preparation of Fmoc-Pro-Gly-Gly-Gly-Gly-OH
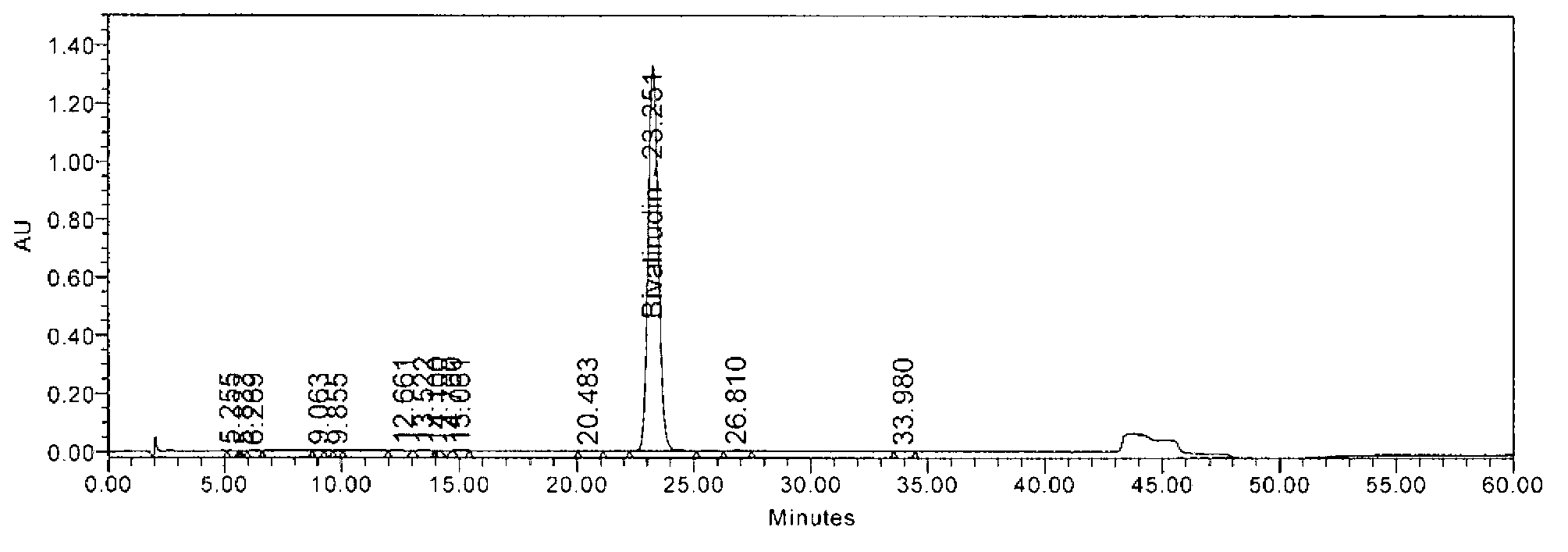
[0062] Weigh tetrapolyglycine (24.6g, 100mmol) and sodium carbonate (21.2g, 200mmol) into 500ml water, stir for 10 minutes to dissolve, add Fmoc-Pro–OSu (47.7g, 110mmol) of 1,4-dioxane The solution was 500ml, stirred and reacted at room temperature for 2.5 hours, TLC detected the end of the reaction, concentrated under reduced pressure to remove 1,4-dioxane, adjusted the pH of the aqueous phase with 2N hydrochloric acid, and when the pH was 2-3, a large amount of solids were precipitated, filtered with suction, Washed with water, dried, and purified by column chromatography to obtain 51.2 g of the target product with a yield of 90.0% and a purity of 98.7%. MS m / z: 566 (M+1). [α] D 20℃ =-24.3°(DMF,c=1.0).
[0063] h 1 NMR (600MHz, CD 3 SOCD 3 ):
[0064] δ8.34(dd,J=4.8Hz,2H),8.24(dd,J=6.0Hz,1H),8.21(dd,J=6.0Hz,1H),8.11-8.16(m,3H),8.06( dd,J=5.4Hz,1H),7.90(q,4H),7.68(t,J=9.0Hz,2H),7.64(d,J=7.8Hz,1H),7...
Embodiment 2
[0066] Example 2, preparation of side chain fully protected bivalirudin peptide-resin
[0067] Weigh 25.0g of Fmoc-Leu-Wang resin (loading capacity 0.4mmol / g, 10mmol) into the solid phase reactor, add 150ml of 20% piperidine DMF solution, stir and react at 25-30°C for 10min, repeat deprotection 3 times , suction filtration after completion of the reaction, the resin was washed with 200ml DMF, suction filtration, repeated washing 6 times, Fmoc-Tyr ( t Bu)-OH (MW: 459,30mmol) 13.8g, 1-hydroxybenzotriazole (HOBt) (HOBt) (MW: 135.1,30mmol) 3.9g were dissolved in 100ml DMF, added to the solid phase reactor, and then N,N - Diisopropylcarbodiimide (DIC) (MW: 126.2, 30mmol) 3.6ml, react at 25-35°C for about 2 hours, the end point of the reaction is subject to the negative test of ninhydrin, repeat the above steps, follow the The rutin peptide sequences are coupled with the corresponding Fmoc protected amino acids one by one, and the molar equivalents of the protected amino acids and ...
Embodiment 3
[0069] Example 3, preparation of side chain fully protected bivalirudin peptide-resin
[0070] Weigh 20.0g of Fmoc-Leu-Wang resin (loading capacity 0.5mmol / g, 10mmol) into the solid phase reactor, add 150ml of 20% piperidine DMF solution, stir and react at 25-30°C for 10min, repeat deprotection 3 times , suction filtration after completion of the reaction, the resin was washed with 200ml DMF, suction filtration, repeated washing 6 times, Fmoc-Tyr ( t Bu)-OH (MW:459,30mmol) 13.8g, N-hydroxy-7-azabenzotriazole (HOAt) (MW:136.1,30mmol) 4.1g, 2-(7-aza-1H- Benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU) (MW:380.2,30mmol) 11.4g was dissolved in DMF and added to the solid phase reactor, Add 5ml of N-methylmorpholine (NMM) (MW: 101.2, ρ: 0.92g / ml, 45mmol) and react for about 2 hours. The rutin peptide sequence is coupled with the corresponding Fmoc protection or Boc protection amino acid one by one, and the molar equivalent of each Fmoc protection or Boc pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com