Bivalirudin freeze-dried injection and preparation thereof
A technology of freeze-dried powder injection and bivalirudin, which is applied in the field of medicine, can solve the problems of variability of active ingredients, obvious degradation of bivalirudin, high impurity content, etc., achieve good stability of the preparation, improve stability and clinical application The effect of stability, good resolubility and compatibility stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
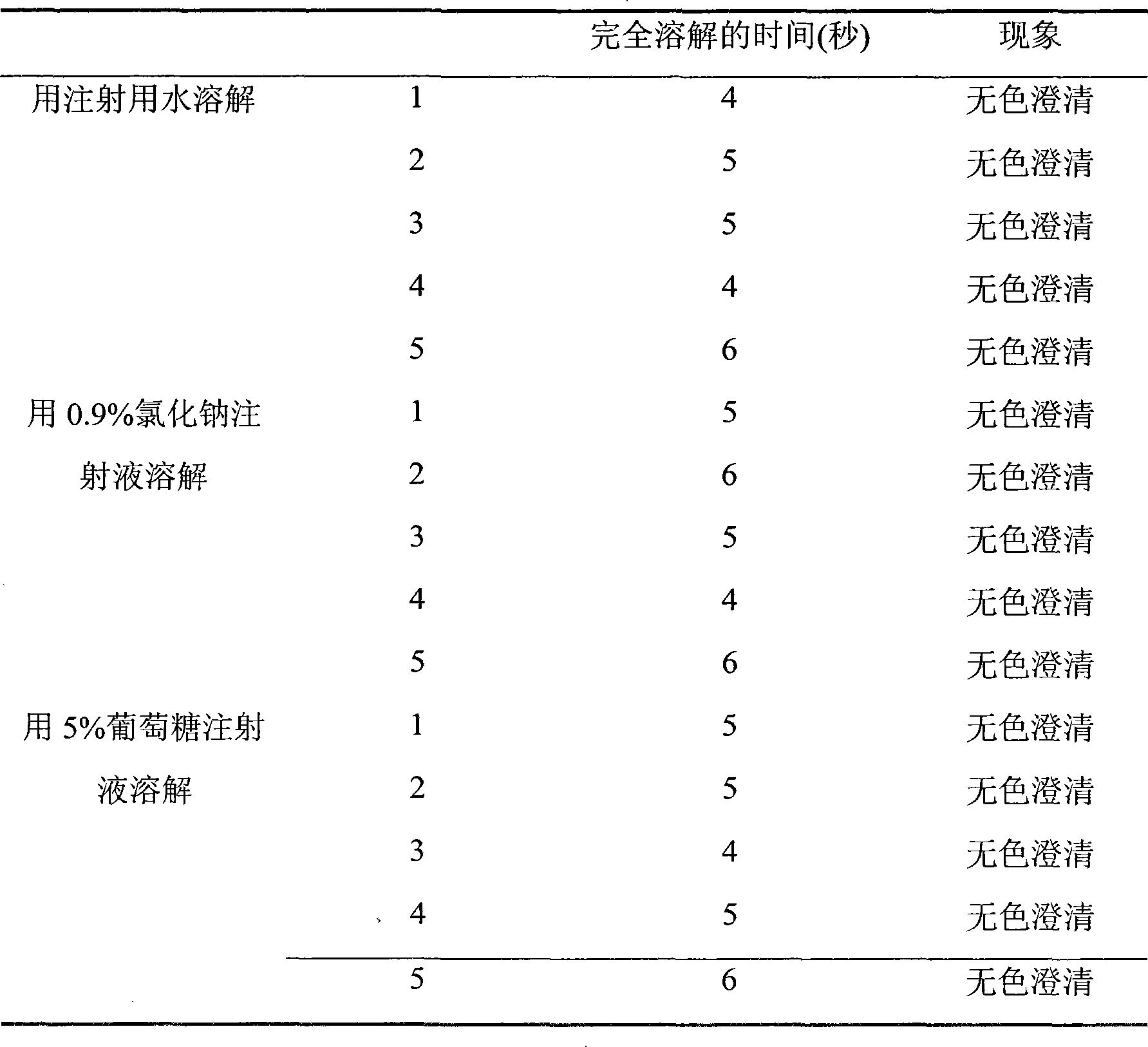
Examples
Embodiment 1
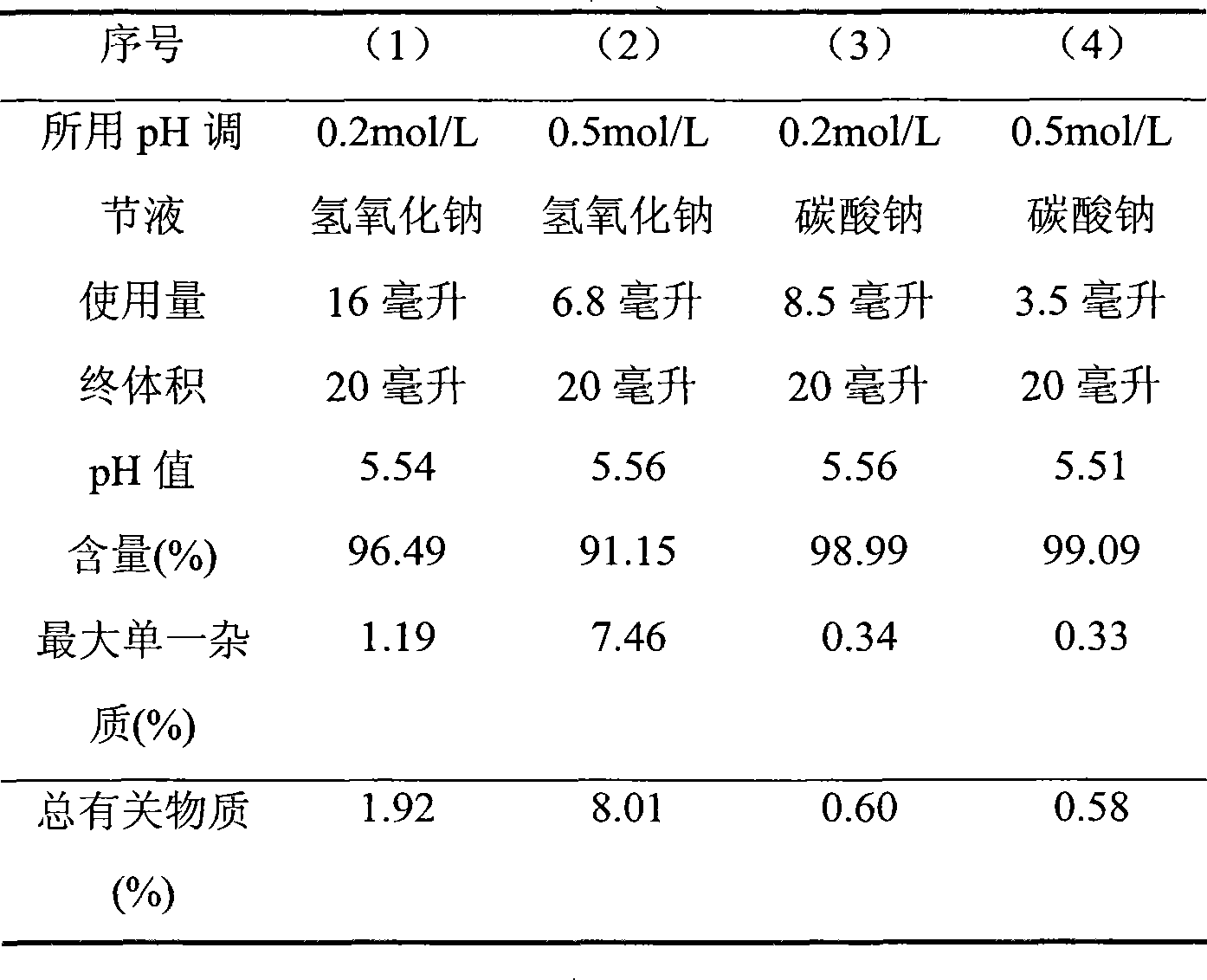
[0019] The following tests were all prepared according to 20 preparations (20 ml volume).
[0020] (1) Take 1.3641g of bivalirudin crude drug, dissolve it with a small amount of water for injection, add 0.2mol / L sodium hydroxide to adjust the pH to about 5.30, add water to 20ml, freeze-dry, and take a sample to determine the relevant properties of this product. substance and content.
[0021] (2) Take 1.3635 g of bivalirudin raw material, dissolve it with a small amount of water for injection, add 0.5 mol / L sodium hydroxide to adjust the pH to about 5.33, add water to 20 ml, freeze-dry, and take a sample to determine the relevant properties of this product. substance and content.
[0022] (3) Take 1.3644g of bivalirudin crude drug, dissolve it with a small amount of water for injection, add 0.2mol / L sodium carbonate to adjust the pH to about 5.32, add water to 20ml, freeze-dry, and take samples to determine the related substances of this product and content.
[0023] (4) Ta...
Embodiment 2
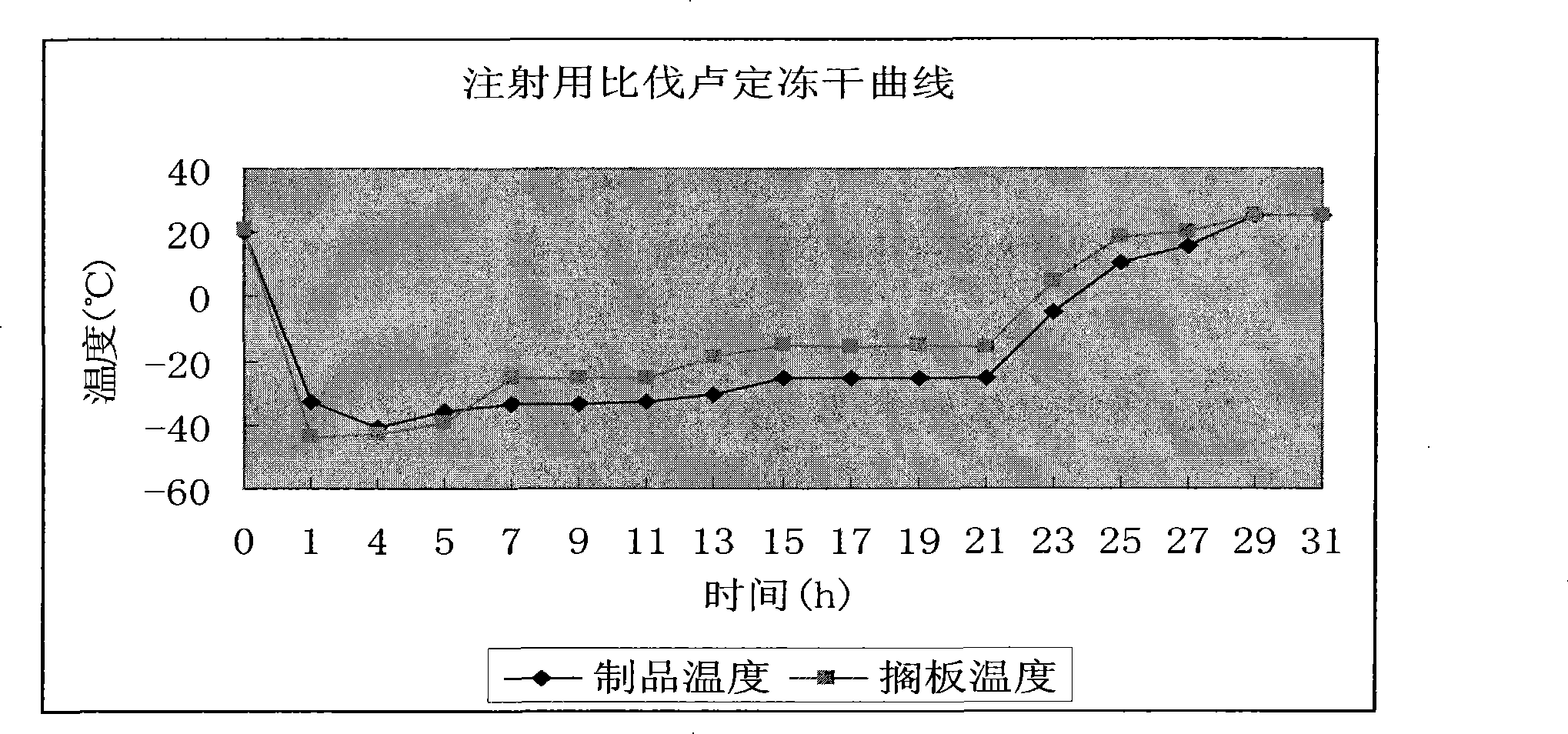
[0029] Take 60.0 g of bivalirudin and 30.0 g of mannitol, dissolve them in 500 ml of water for injection, adjust the pH to 4.5-6.5 with 0.5 mol / L sodium carbonate solution, dilute to 1000 ml, and stir evenly. Sterile filtration with 0.22μm membrane, subpackaging, semi-stoppering, freeze-drying, stoppering, and capping to obtain the product with a specification of 60mg. Freeze-drying curve such as figure 1 shown.
Embodiment 3
[0031] Take 60.0 g of bivalirudin and 30.0 g of dextran, dissolve them in 500 ml of water for injection, adjust the pH to 4.5-6.5 with 0.5 mol / L sodium carbonate solution, dilute to 1000 ml, and stir evenly. Sterile filtration with 0.22μm membrane, subpackaging, semi-stoppering, freeze-drying, stoppering, and capping to obtain the product with a specification of 60mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com