Method for synthesis of bivalirudin in solid-phase fragment approach
A technology of bivalirudin and synthesis ratio, applied in the field of solid-phase fragment method synthesis of peptides, can solve the problems of difficult large-scale production, difficult product purification, low product purity, etc., and achieves cost increase, less environmental pollution and high yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
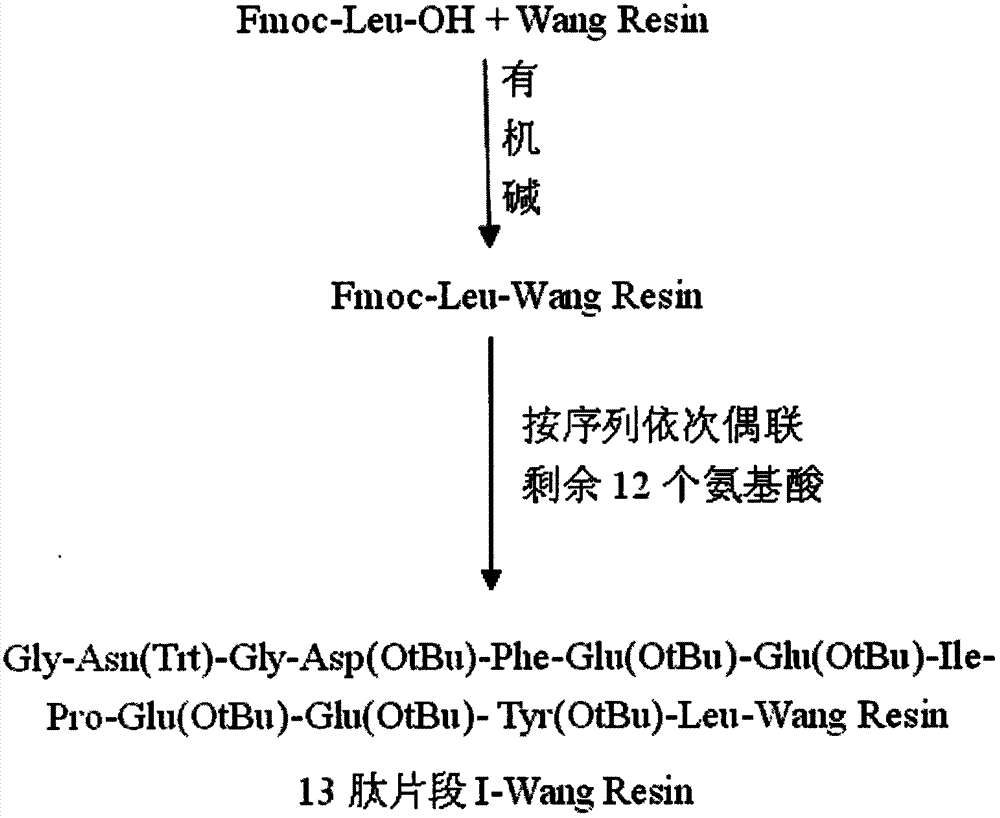
[0026] Preparation of 13 Peptide Fragment I-Wang Resin
[0027] Weigh 1g of Wang Resin with a substitution degree of 0.7mmol / g, add it to a solid-phase reaction column, add anhydrous 10ml DCM to swell for 30min, wash with anhydrous DMF 3 times (1min each time), and add 1.4mmol of Fmoc -Leu-OH and HOBt were dissolved in 5ml of anhydrous DMF, then 1.4mmol of DIC was added to activate at 0-5°C for 5min, then added to a solid-phase reaction column for 10min, then 0.2mmol of DMAP was added, and reacted at 30°C for 5h. After completion, use anhydrous DMF and CH respectively 3 Wash with OH, DCM, DMF 2, 1, 1, 2 times, each time not less than 1min, and the detection substitution is 0.65mmol / g. Use 0.1 mmol acetic anhydride and pyridine to block unreacted hydroxyl groups on the resin for 1 h to obtain Fmoc-Leu-Wang.
[0028] After removing the Fmoc protecting group on Fmoc-Leu-Wang with 10ml volume of 25% piperidine-DMF in turn (the first reaction was 5 minutes, the middle was washed ...
Embodiment 2
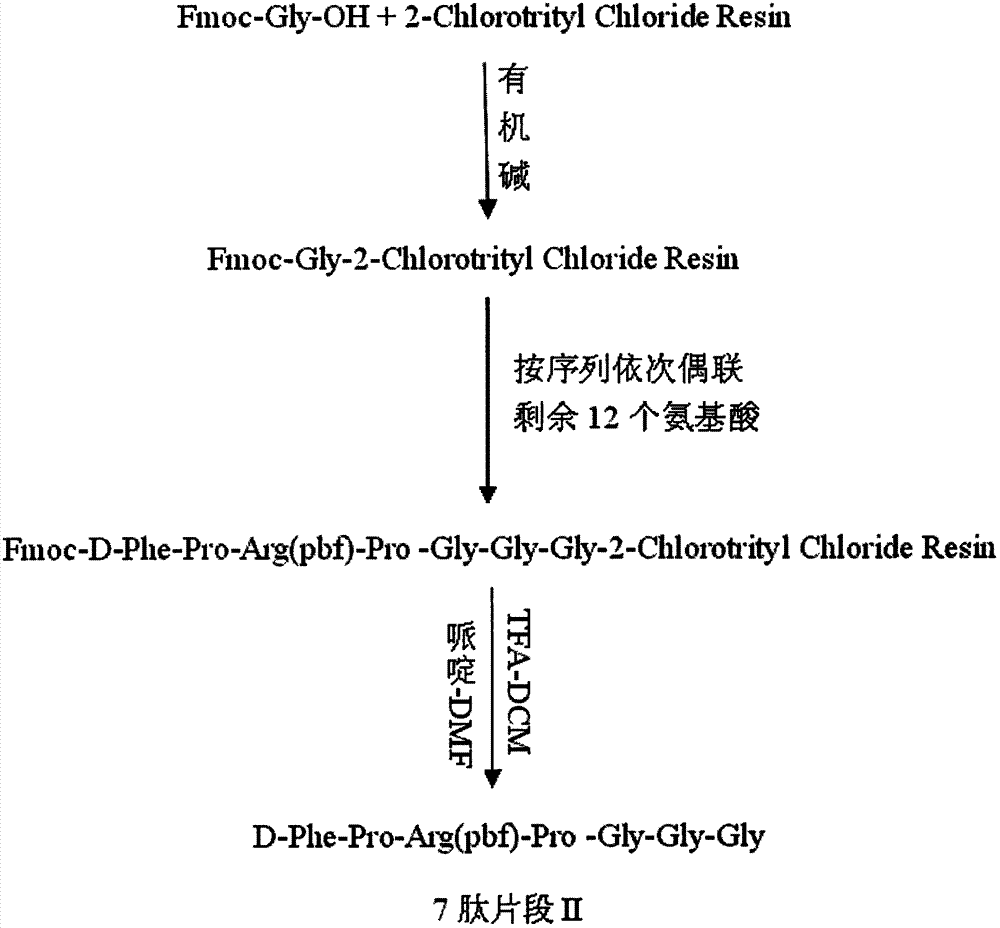
[0030] Preparation of 7-peptide Fragment II
[0031] Weigh 2g of 2-Chlorotrityl Chloride Resin with a degree of substitution of 0.7mmol / g, add it to a solid-phase reaction column, add anhydrous DCM to swell for 30min, wash with 10ml anhydrous DMF 3 times (1min each time), add 2.4 One mmol of Fmoc-Gly-OH and HOBt were dissolved in 5ml of anhydrous DMF, and then 1.4mmol of DIC was added to activate for 5min at 0-5°C, then added to the solid-phase reaction column for 10min, then 0.4mmol of DMAP was added, and the React at ℃ for 5 hours, wash twice with 10ml of anhydrous DMF, each time for 1min, and detect the substitution of 0.68mmol / g. Use 0.2mmol acetic anhydride and pyridine to block unreacted amino groups on the resin for 1h to obtain Fmoc-Gly-2-Chlorotrityl Chloride Resin. Use 20ml volume of 25% piperidine / DMF to remove the Fmoc protecting group on Fmoc-Leu-Wang for 2 times (the first reaction is 5min, the second reaction is 10min, the middle is washed once with 10ml DMF), ...
Embodiment 3
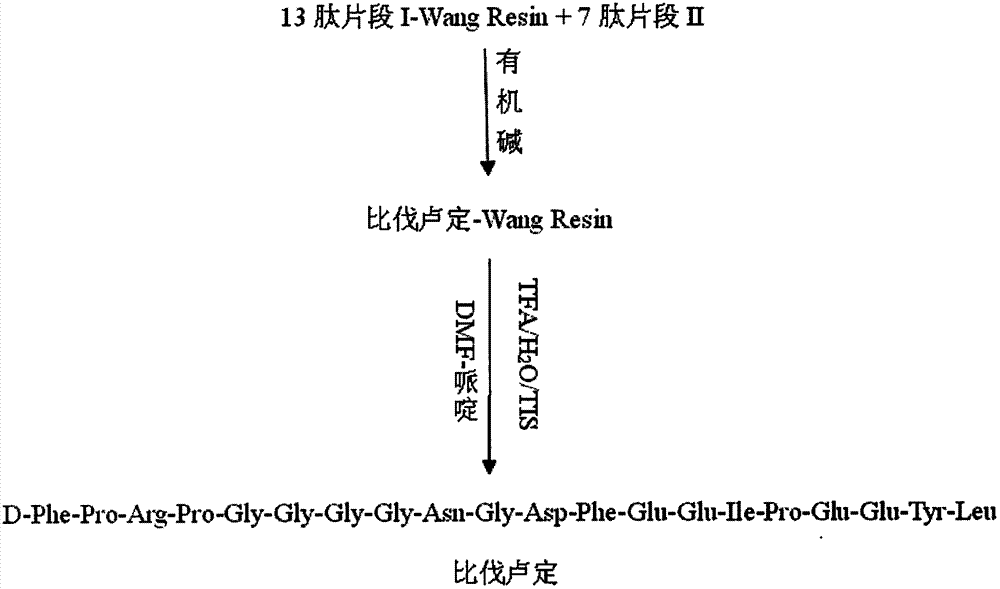
[0033] Synthesis of Bivalirudin by Solid Phase Fragmentation
[0034] Take a 1.5-fold excess of 7-peptide fragment II, a 2-fold excess of HOBt, HUBT, and anhydrous DMF dissolved in 0-5°C, add a 1.5-fold excess of DIEA to activate for 5 minutes, and add it to a solid-phase synthesis column. React at 30°C for 1-5h (the end point of the reaction is determined by the ninhydrin method), wash with anhydrous DMF for 3 times, dalirudin-Wang Resin (D-Phe-Pro-Arg(pbf)-Pro-Gly-Gly -Gly-Gly-Asn(Trt)-Gly-Asp(OtBu)-Phe-Glu(OtBu)-Glu(OtBu)-Ile-Pro-Glu(OtBu)-Glu(OtBu)-Tyr(OtBu)-Leu- Wang Resin). After adding 15ml of 25% piperidine-DMF as the Fmoc deprotecting agent for 2 times (the first reaction is 5min, the second reaction is 10min, and the middle is washed once with 10ml DMF), sequentially wash with DMF, DCM, CH 3 Wash 2, 1, and 3 times with OH, each time not less than 2 min; add 15 ml of TFA / H with a volume ratio of 95:2.5:2.5 2 O / TIS was used as a reagent for cleavage and removal of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com