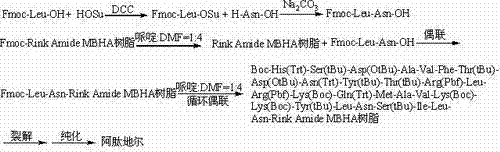
A kind of method for preparing alendidil
A synthesis method and technology of peptide fragments, applied in the field of polypeptide drug preparation, can solve problems such as low synthesis efficiency, unsuitability for large-scale production, and high cost of histidine racemization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Synthesis of Fmoc-Leu-OSu Activated Ester
[0087] Weigh 353.41g Fmoc-Leu-OH (1.0mol), add 138.10g HOSu (1.2mol) into 2000ml THF, add 247.56g DCC (1.2mol) under ice-water bath, react for 1 hour, warm up to room temperature for 3 hours, and react liquid filtration, mother liquor was spin-dried, dissolved in DCM, filtered, washed 3 times with saturated sodium bicarbonate, 2 times with pure water, back-extracted 2 times, combined organic phase, dried with anhydrous sodium carbonate, spin-dried, and recrystallized 3 times with ice ethanol , filtered, and the solid oil pump was dried to obtain 400.95g Fmoc-Leu-OSu activated ester, yield 89%.
Embodiment 2
[0089] Synthesis of Fmoc-Leu-Asn-OH
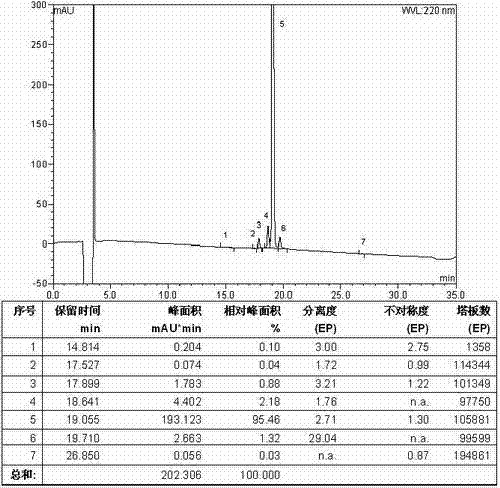
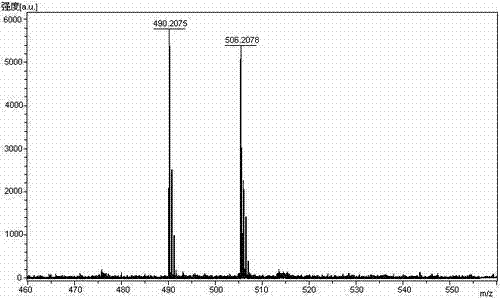
[0090] Weigh 66.06g H-Asn-OH (0.5mol) and 79.50g Na 2 CO 3 (0.75mol) was added to the mixed solution of 500ml water and 500ml THF to dissolve, weighed 225.25g Fmoc-Leu-OSu (0.5mol) was added to 500ml THF, dissolved and added dropwise to the above mixed solution, reacted overnight at room temperature, with Adjust the pH to 7 with 10% dilute hydrochloric acid, remove THF by rotary evaporation, and then adjust the pH to 3. A large white precipitate was obtained which was filtered. The resulting white precipitate was recrystallized from ice ethanol. The solid oil pump was dried to obtain 203.38g Fmoc-Leu-Asn-OH, the yield was 87%. Its HPLC spectrum is as figure 2 Shown, HPLC purity 95.46%, yield 89%. Its mass spectrum is as image 3 Shown, [M+Na] + : 490.2075, [M+K] + : 506.2078, the theoretical exact molecular weight of the compound dipeptide fragment Fmoc-Leu-Asn-OH is: 467.2056, the mass spectrometry result of the sample is consis...
Embodiment 3
[0092] Synthesis of Altideil-Rink Amide MBHA Resin
[0093] Weigh 100 g of Rink Amide MBHA resin with a degree of substitution of 0.10 mmol / g, add it to a solid-phase reaction column, wash it once with DMF, and swell the resin with DCM for 30 minutes, then use piperidine and The deprotection solution composed of DMF removes the Fmoc protecting group on the amino resin, washes it 6 times with DMF, takes 14.03g Fmoc-Leu-Asn-OH (30mmol), 4.05g HOBt (30mmol) and dissolves it in DMF, and adds 3.88 After g DIC (30mmol) is activated, add in the reaction column that above-mentioned resin is housed, after reacting at room temperature for 2 hours, detect and judge the end of the reaction with the ninhydrin method, if the resin is colorless and transparent, it means that the reaction is complete; the resin develops color, It means that the reaction is not complete and needs to be reacted for another 1 hour. This judgment standard is applicable to the ninhydrin method detection and judgme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com