Synthetic method of decarboxylated carnosine
A technology of decarboxylated carnosine and a synthesis method, which is applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of large environmental pollution, strong catalyst toxicity, and many process steps, achieve high purity and total yield, and reduce the cost of protective groups. , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1Bo
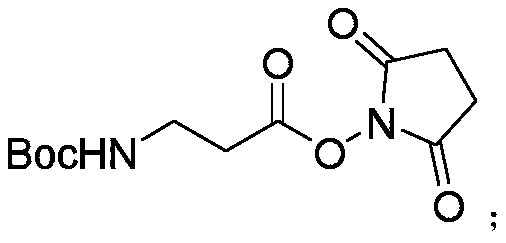
[0031] Example 1 Synthesis of Boc-β-Ala-OSU
[0032] Prepare a 3L serum bottle and wash it twice with 200mL DCM.
[0033] Add 189.21g Boc-β-Ala-OH and 138.1g HOSU into the serum bottle, and add 800mL DCM to dissolve. Ice bath, make the system temperature drop below 10℃.
[0034] In addition, 230.03g EDC.HCl was dissolved in 1L DCM, and the resulting solution was slowly added dropwise to the above system.
[0035] After 6 hours of reaction, 11.51g HOSU and 19.17g DEC.HCl were added, and samples were taken after 12 hours of reaction, and the reaction was completed after detection by thin layer chromatography. The raw materials disappeared completely.
[0036] The insoluble matter in the reaction system was removed by filtration, washed with ice water, and extracted with ethyl acetate. The extract was treated with a rotary evaporator and spin-dried to obtain Boc-β-Ala-OSU for use.
Embodiment 2B
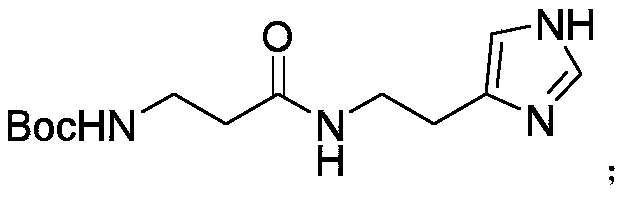
[0037] Example 2 Synthesis of Boc-β-Ala-histamine
[0038] Prepare a 5L serum bottle and wash it twice with 200mL DCM.
[0039] Mix 193.3g histamine dihydrochloride, 420g NaHCO 3 Add to the serum bottle, add 600mL ACN and 600mL water, and stir thoroughly.
[0040] In addition, the intermediate 286.3g Boc-β-Ala-OSU from the previous step was dissolved in 1500mL ACN and slowly added to the above system. After reaction for 2h, the reaction was completed after the raw material point disappeared completely after detection by thin layer chromatography.
[0041] After filtration, the filtrate was treated with a rotary evaporator to remove ACN, and a large amount of solids were precipitated. After being drained, Boc-β-Ala-histamine was obtained for use.
Embodiment 3
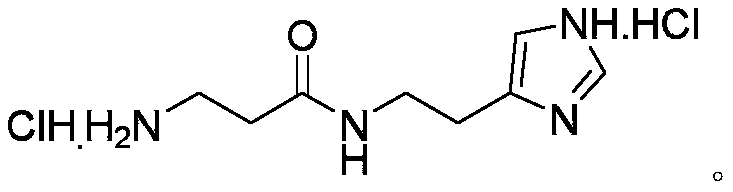
[0042] Example 3 Deprotection
[0043] Add the solid from the previous step to EA, stir well, add HCl / methyl acetate, after 40 minutes of reaction, remove the liquid by suction filtration, and wash the solid with EA foam 3 times. The finished product decarboxylized carnosine is obtained by draining, with a purity of 99.2% and a total yield of 69.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com