Preparation method of Tirzepatide
A fmoc-tyr-ser, boc-tyr technology, applied in the field of peptide drug preparation, can solve the problems of unfavorable industrial production, low purity and yield, high cost, etc. Effect of material cost and purification cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation method of Tirzepatide
[0021] Synthesis of Tirzepatide Peptide Resin
[0022] Tirzepatide Peptide Resin:
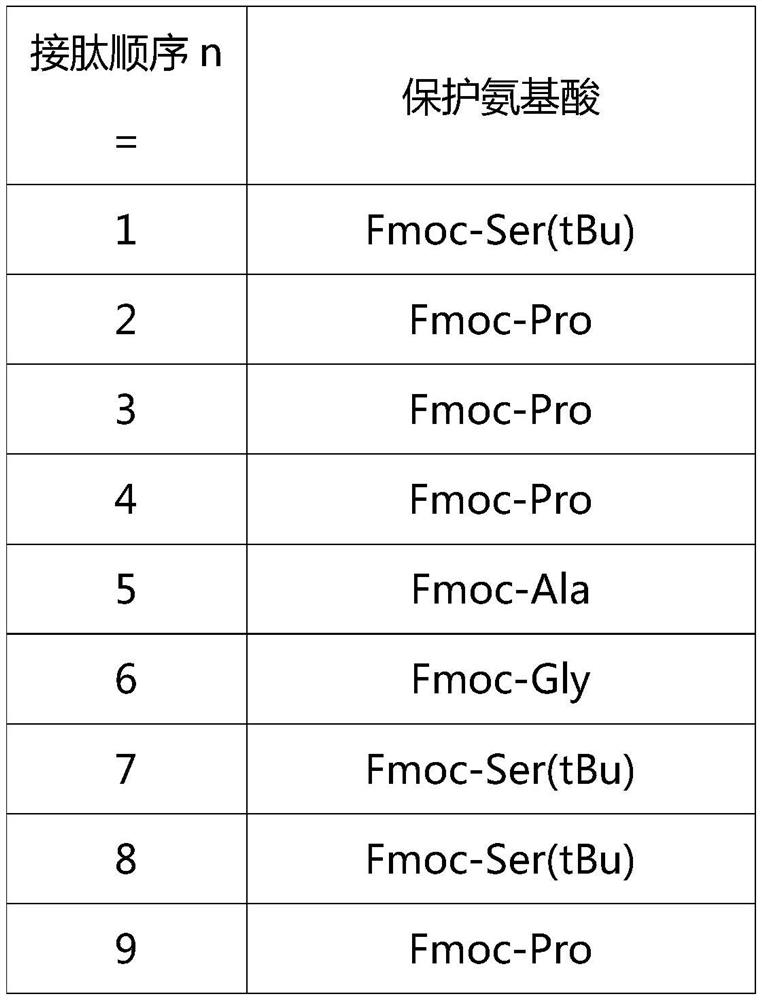
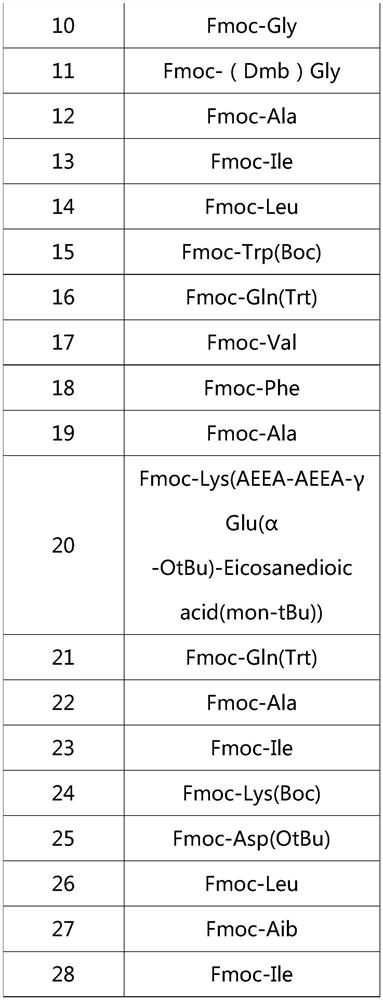
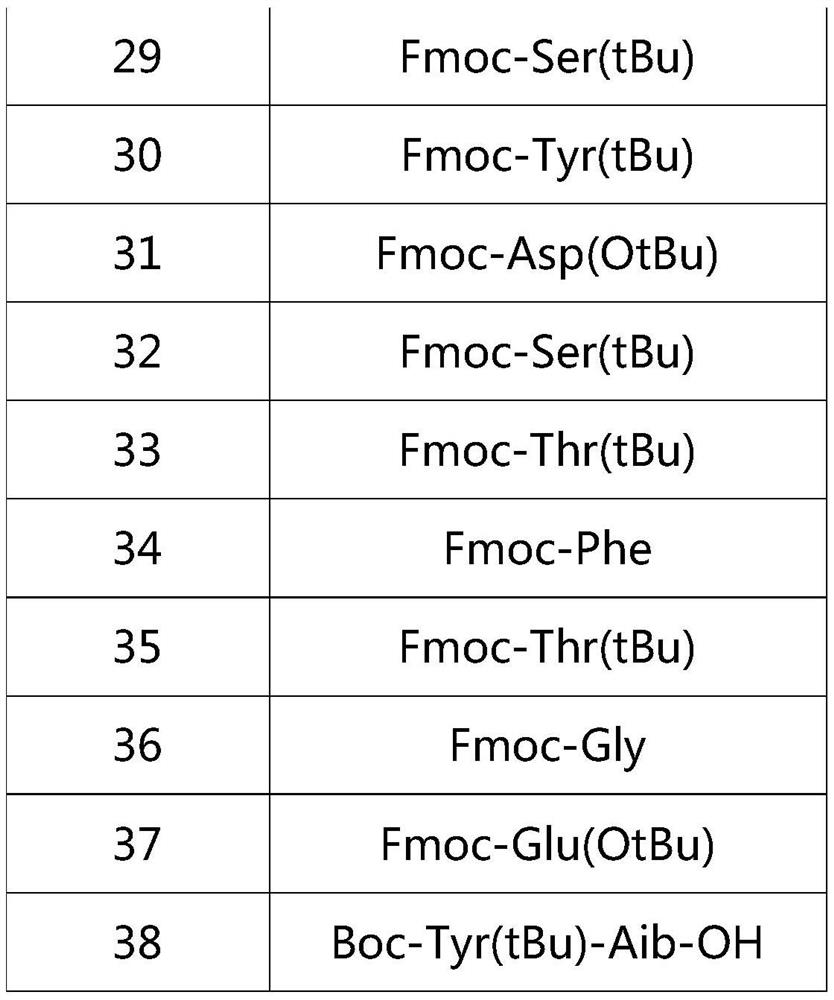
[0023] Boc-Tyr(tBu)-Aib-Glu(OtBu)-Gly-Thr(tBu)-Phe-Thr(tBu)-Ser(tBu)-Asp(OtBu)-Tyr(tBu)-Ser(tBu)-Ile- Aib-Leu-Asp(OtBu)-Lys(Boc)-Ile-Ala-Gln(Trt)-Lys(AEEA-AEEA-γGlu-Eicosanedioicacid(mon-tBu))-Ala-Phe-Val-Gln(Trt)- Trp(Boc)-Leu-Ile-Ala-Gly-Gly-Pro-Ser(tBu)-Ser(tBu)-Gly-Ala-Pro-Pro-Pro-Ser(tBu)-Aminoresin.
[0024] Using Rink Amide MBHA resin as the starting resin, the Tirzepatide peptide resin was prepared by de-Fmoc protection and coupling reaction, followed by coupling with the protected amino acids in Table 1, Table 2, and Table 3. The protected amino acids or fragments corresponding to the protected amino acids used in this example are as follows:
[0025] Table 1
[0026]
[0027]
[0028]
[0029] Table 2
[0030]
[0031]
[0032]
[0033] table 3
[0034]
[0035]
[0036]
[0037]1. Insert th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com