A method for synthesizing amino acid n-methylation and its product and application
A methylation and amino acid technology, applied in the preparation of cyanide reaction, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of poor industrialization prospects, many reaction steps, and highly toxic reagents, and achieve structural pertinence. Strong, low cost, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
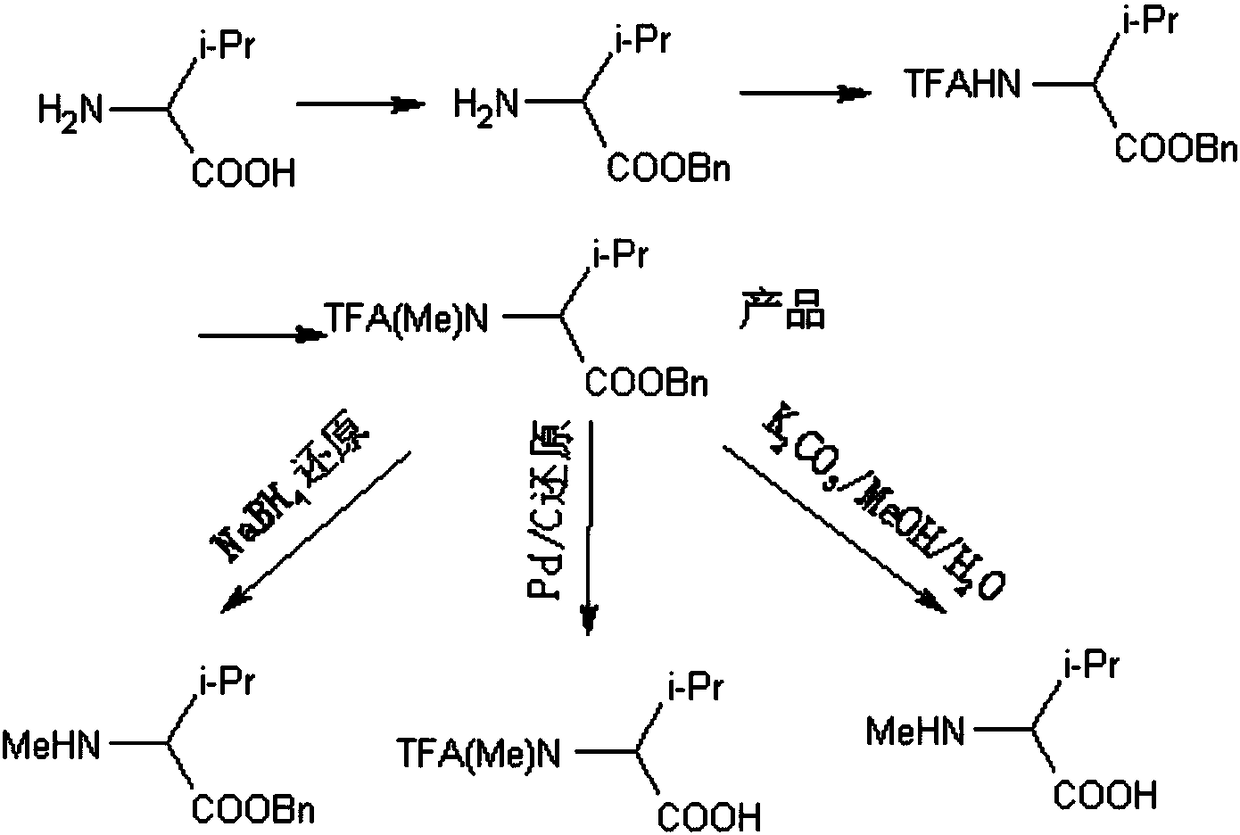
[0034] A method for synthesizing amino acid N-methylation, the specific reaction scheme refers to figure 1 .
[0035] In particular, N-methyl(trifluoroacetyl)-amino acid benzyl ester has not been reported, and it needs to be prepared by methylation of N-trifluoroacetyl-amino acid benzyl ester according to the present invention.
[0036] In particular, there are no reports on N-trifluoroacetyl-amino acid benzyl esters, but according to the present invention, amino acid benzyl esters are prepared by trifluoroacetylation protection.
[0037] In particular, when amino acid benzyl ester is not a product that has already been purchased, it is produced from amino acid through esterification.
[0038] Specifically, taking valine as an example to prepare N-methyl (trifluoroacetyl)-valine benzyl ester, the operation is as follows:
[0039] (1) Obtain valine benzyl ester from valine through nucleophilic reaction
[0040] Specifically, in this example, valine is used to synthesize vali...
Embodiment 2
[0049] The N-methyl (trifluoroacetyl)-amino acid benzyl ester prepared by the present invention can be obtained by NaBH 4 N-methyl-amino acid benzyl ester can be obtained by reduction; N-methyl (trifluoroacetyl)-amino acid can be obtained by Pd / C hydrogenation reduction; it can be obtained by K 2 CO 3 / MeOH / H 2 Deprotection of O gives N-methyl-amino acid, which can be selected according to the actual use of N-methyl(trifluoroacetyl)-amino acid benzyl ester.
[0050] Specifically, taking the N-methyl(trifluoroacetyl)-valine benzyl ester prepared in Example 1 as an example, it is hydrogenated and reduced by Pd / C to obtain N-methyl(trifluoroacetyl)-valine Valine.
[0051] Specifically, in this example, N-methyl(trifluoroacetyl)-valine was synthesized using N-methyl(trifluoroacetyl)-valine benzyl ester, and the experimental steps were: 100mL single-necked flask 2.00 g (0.0063 mol) of N-methyl(trifluoroacetyl)-valine benzyl ester prepared in Example 1 was added, tetrahydrofuran...
Embodiment 3
[0054] (1) Obtain alanine benzyl ester through nucleophilic reaction from alanine
[0055] Specifically, in this example, alanine was used to synthesize benzyl alanine, and the experimental procedure was as follows: in a 100mL single-necked flask, 2.00g of alanine (22.4mmol), 3.71g of K 2 CO 3 (1.2eq.26.9mmol, add 50mL acetone, stir at 35°C for 30min, add tetrabutylammonium bromide, nitrogen protection, dropwise add 2.68g benzyl chloride (0.95eq.21.2mmol) acetone (10mL) mixture, react for 12h. After the reaction, suction filtration, the filtrate was spin-dried at 35°C, dissolved in dichloromethane, washed with a saturated aqueous sodium chloride solution, the organic layer was dried with anhydrous sodium sulfate, suction filtration, the filtrate was spin-dried at low temperature, and separated by column chromatography [V (petroleum Ether): V (ethyl acetate) = 4: 1] to obtain 3.07 g of a colorless oily liquid with a yield of 76.5%.
[0056] (2) Obtain N-trifluoroacetyl-alanin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com