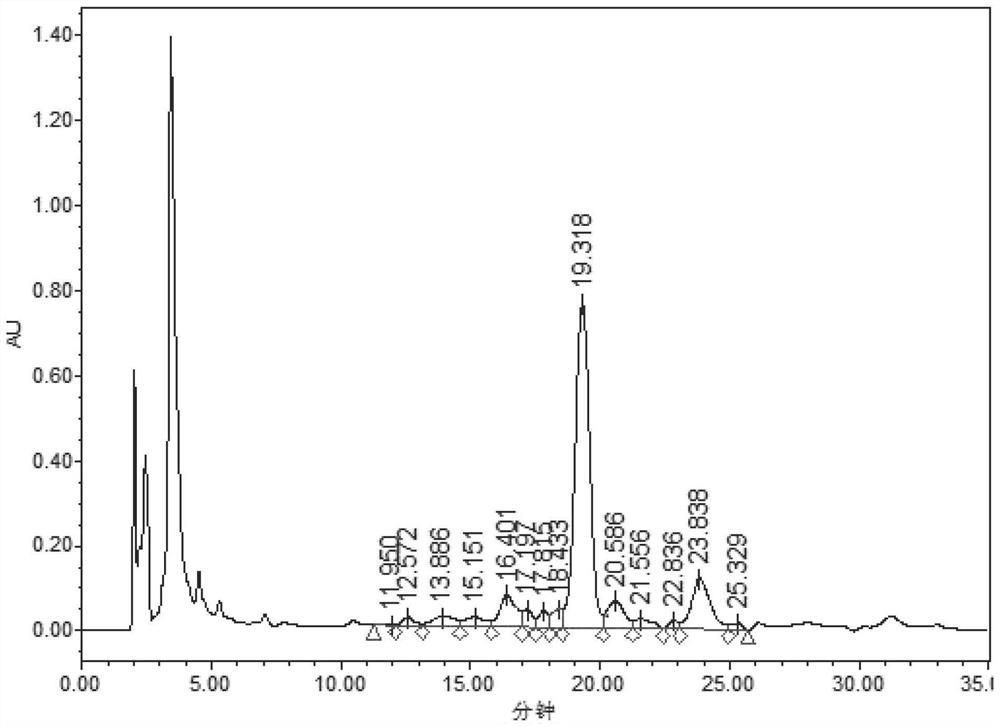
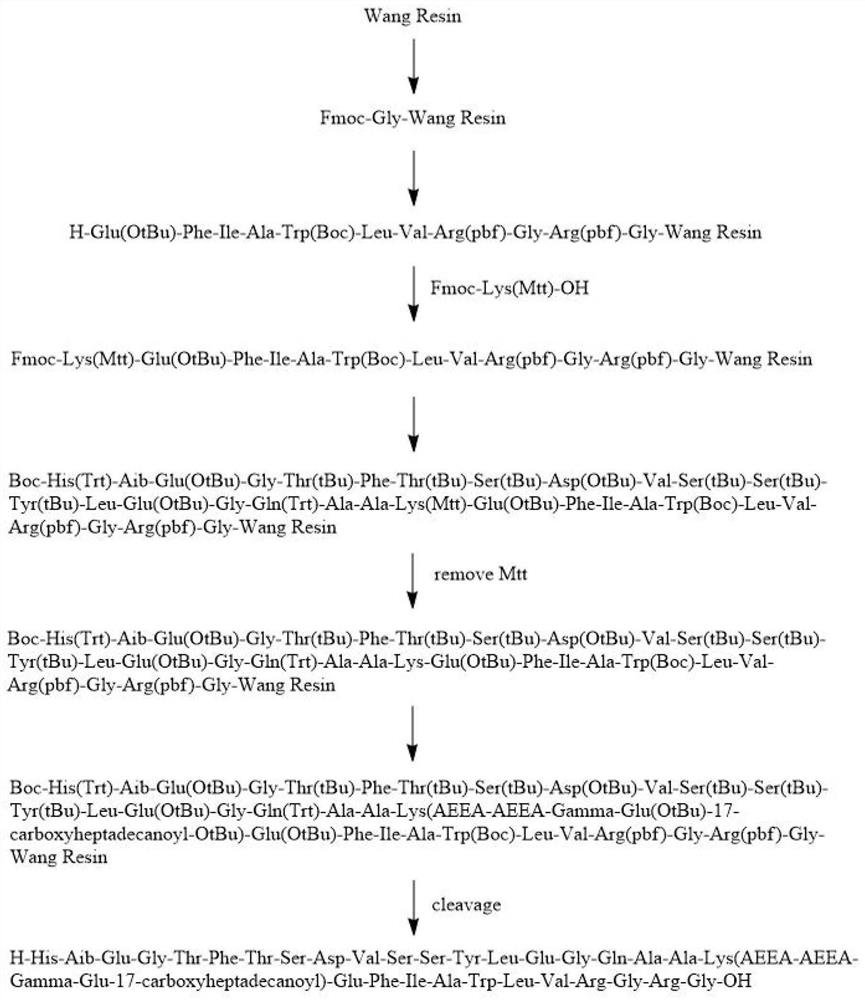
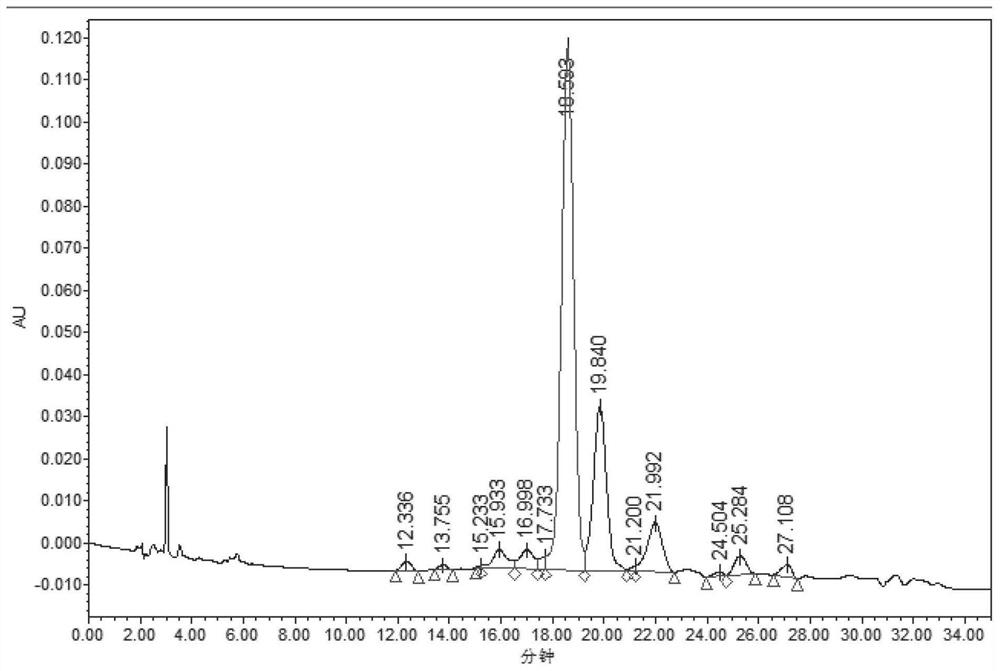
Preparation method of somaglutide
A peptide resin and resin technology, applied in the field of peptide synthesis, can solve the problems of increasing the difficulty of side chain coupling, high production costs, and increasing the amount of racemized impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The invention provides a preparation method of semaglutide, comprising the following steps:
[0031] (1) Mix the starting resin, Fmoc-Gly-OH, pyridine catalyst, coupling reagent and solvent, and perform the first coupling reaction and capping resin in sequence to obtain Fomc-Gly-resin;
[0032] (2) Fmoc-Arg (pbf)-OH is connected on the Fomc-Gly-resin to obtain Fomc-Arg (pbf)-Gly-resin; the connection includes the removal of the Fomc protecting group reaction and the second coupling reaction;
[0033] (3) According to the method of step (2), connect Fmoc-Gly-OH, Fmoc-Arg(pbf)-OH, Fmoc-Val-OH, Fmoc-Leu-OH, Fmoc-Trp(Boc)-OH, Fmoc-Ala-OH, Fmoc-Ile-OH, Fmoc-Phe-OH, Fmoc-Glu(OtBu)-OH and R 1 -Lys(R 2 )-OH, to obtain the first peptide resin; the R 1 Including Fmoc, Mtt, Mmt, ivDde, Dde or Alloc; the R 2 Including Fmoc, Mtt, Mmt, ivDde, Dde or Alloc; the structural formula of the first peptide resin is R 1 -Lys 26 (R 2 )-Glu(OtBu) 27 -Phe 28 -Ile 29 -Ala 30 -Trp(Boc...
Embodiment 1
[0085] (1) Add 17.25g Wang resin with a substitution degree of 0.58mmol / g to the synthesis reactor, add 80mL DMF to swell the resin for 60min; dissolve 8.94g Fmoc-Gly-OH, 4.08g HOBT and 0.611g Dmap with 20mL DMF , placed in an ice-water bath and mixed for 20 minutes, added 4.62mL DIC and stirred at room temperature for 5 minutes to obtain a mixed solution; the obtained mixed solution was added to the above-mentioned synthesis reactor, and the first coupling reaction was performed at room temperature for 2.5 hours, filtered, and the obtained Wash the solid component with DMF 5 times, add 100mL acetic anhydride / NMM / DMF (the concentration of acetic anhydride is 20%, and the concentration of NMM is 12%) to cap the resin for 3 hours, wash with DMF twice, methanol twice, dichloromethane twice After washing with methanol for 2 times, it was sucked dry to obtain Fmoc-Gly-resin (19.95g, 9.78mmol, degree of substitution: 0.49mmol / g, yield: 99.70%).
[0086] (2) Add 10.21 g of the Fmoc-G...
Embodiment 2
[0107] (1) Add 34.5g Wang resin with a substitution degree of 0.58mmol / g to the synthesis reactor, add 300mL DMF to swell the resin for 60min; dissolve 5.96g Fmoc-Gly-OH, 2.72g HOBT and 1.22g Dmap with 80mL DMF , placed in an ice-water bath and mixed for 20 minutes, added 3.08mL DIC and stirred at room temperature for 5 minutes to obtain a mixed solution; the obtained mixed solution was added to the above synthesis reactor, the first coupling reaction was performed at room temperature for 2.5 hours, filtered, and the obtained The solid component was washed 5 times with DMF, 500 mL of acetic anhydride / NMM / DMF (wherein the concentration of acetic anhydride was 20%, and the concentration of NMM was 11%) was added to cap the resin for 3 hours, washed twice with DMF, washed twice with methanol, dichloromethane After washing twice and twice with methanol, it was sucked dry to obtain Fmoc-Gly-resin (36.42g, 5.90mmol, degree of substitution: 0.16mmol / g, yield: 91.03%).
[0108] (2) Ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com