Amido bond symmetrical molecular tweezers-containing series of porphyrin compounds and preparation method thereof
A technology of porphyrin compounds and symmetrical molecules, which is applied in the fields of 1/11 group organic compounds without C-metal bonds, organic chemistry, color/spectral characteristic measurement, etc., can solve the problems of incomplete detection performance and few types, and achieve The effect of the simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
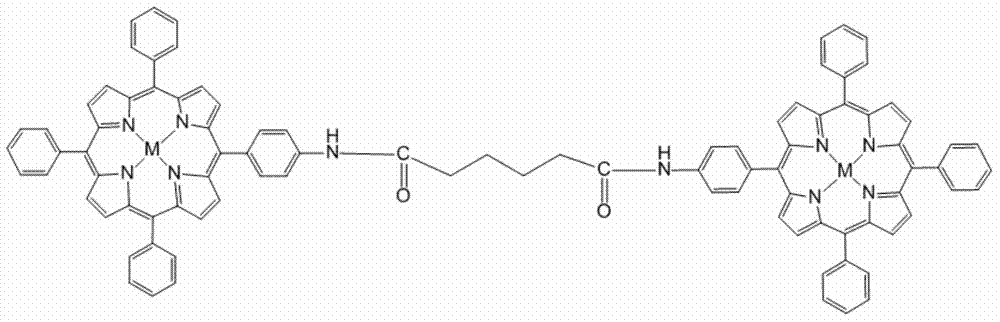
[0035] The molecular tweezer metalloporphyrin compound for detecting benzene of the present invention will be further described in detail in conjunction with specific examples below.
[0036] 1. A porphyrin compound containing amide bond symmetrical molecular clamps.
[0037] The structural formula of the porphyrin compound is as figure 1 Shown:
[0038]
[0039] The molecular clamp porphyrin compound containing amide bond symmetry, when M is 2H, the molecular formula is C 96 h 74 N 10 o2 , referred to as free base porphyrin P.
[0040] The series of metalloporphyrin compounds containing amide bond symmetrical molecular tweezers, M is Zn, Mn, CuCl, Ni or Co collectively referred to as a series of metalloporphyrins;
[0041] When M is Zn, the molecular formula is C 96 h 70 N 10 o 2 Zn 22 ;
[0042] When M is MnCl, the molecular formula is C 96 h 70 N 10 o 2 Cl 2 mn 2 ;
[0043] When M is Cu, the molecular formula is C 96 h 70 N 10 o 2 Cu 2 ;
[0044] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com