Red light organic electrophosphorescence material metal iridium coordination compound and preparation method thereof, and organic electroluminescent device
A phosphorescent material, metal iridium technology, applied in luminescent materials, electric solid devices, organic chemistry and other directions, can solve the problems of affecting the luminous efficiency, small steric hindrance and poor stability of iridium complex phosphorescent materials, and reduce self-efficacy. Quenching phenomenon, less difficulty, and the effect of improving electroluminescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
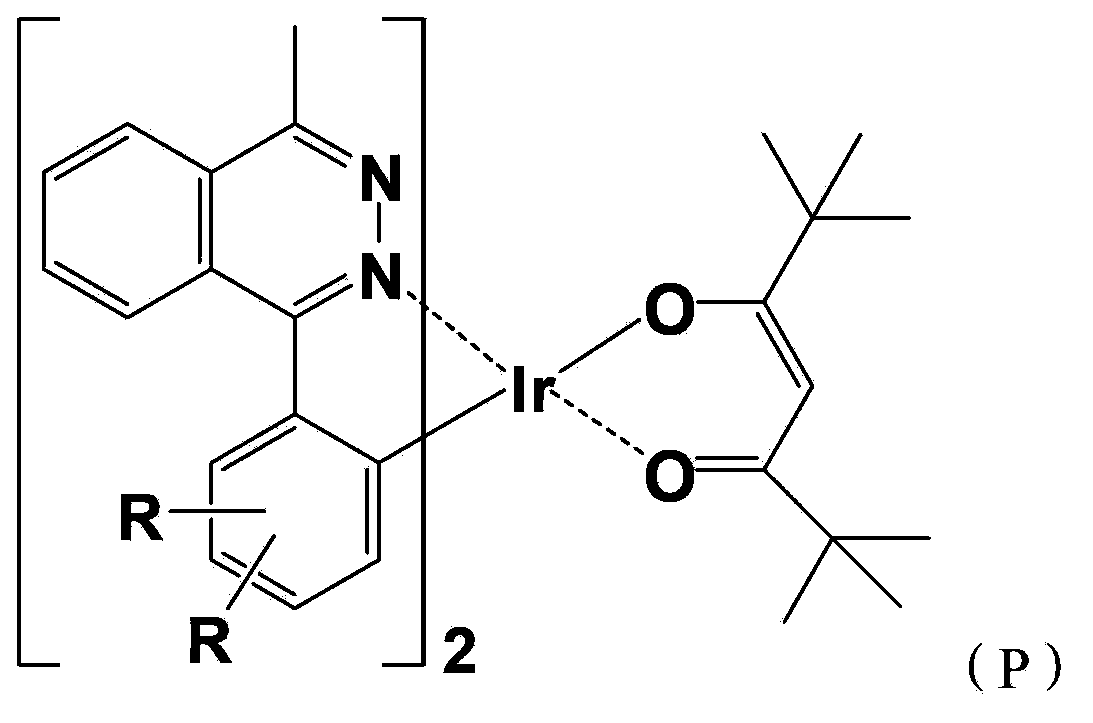
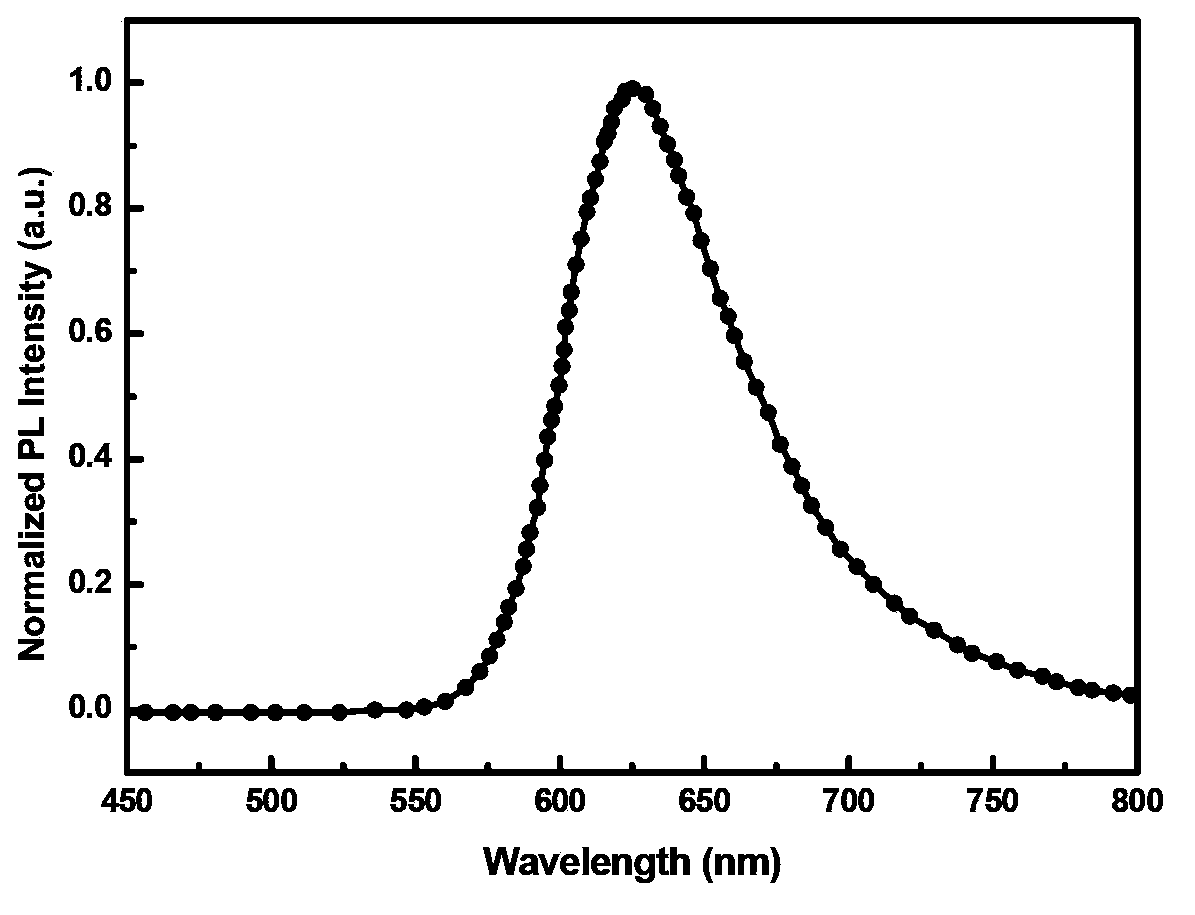
[0059] A red-light organic electrophosphorescent material metal iridium complex bis[1-methyl-4-(4',6'-dimethylphenyl)phthalazine-N,C 2 '](2,2,6,6-tetramethyl-3,5-heptanedione)iridium, with 1-methyl-4-(2',4'-dimethylphenyl)phthalazine as Cyclometallic ligands, the structural formula is as follows:
[0060]
[0061] Wherein, the structural formula of the ring metal ligand is:
[0062]
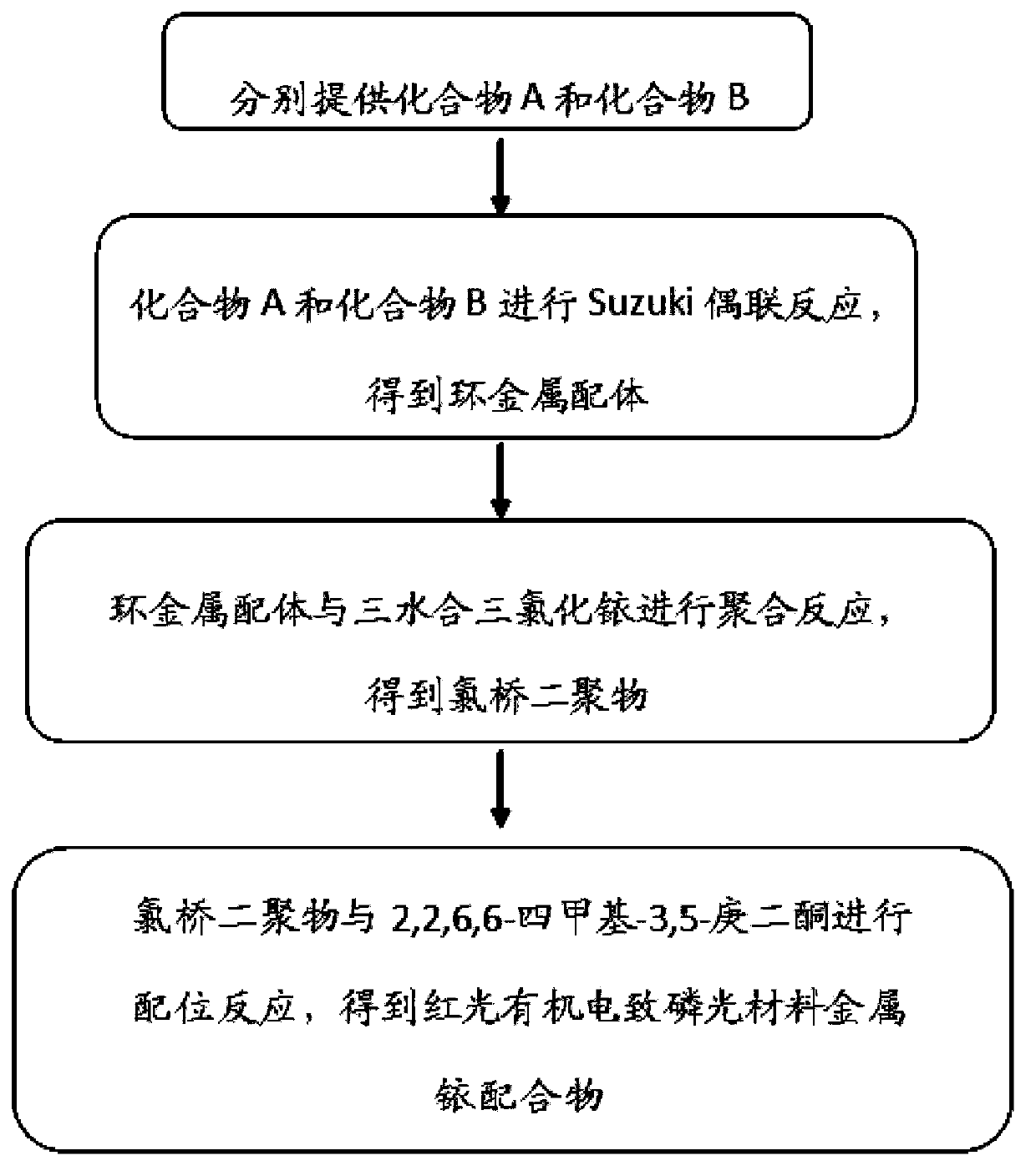
[0063] The preparation method of the metal iridium complex of the above-mentioned red light organic electrophosphorescent material comprises the following steps:
[0064] (1) Provide compound (A) and compound (B1) represented by the following structural formula respectively:
[0065]
[0066] Among them, compound A is 1-methyl-4-chlorophthalazine, and B1 is 2,4-dimethylphenylboronic acid.
[0067] (2) Synthesis of cyclometal ligand 1-methyl-4-(2',4'-dimethylphenyl)phthalazine
[0068]
[0069] Under nitrogen protection, 0.78g (4.0mmol) 1-methyl-4-chlorophthalazine, 0.72g (4.8mmol)...
Embodiment 2
[0091] A red-light organic electrophosphorescent material metal iridium complex bis[1-methyl-4-(5',6'-dimethylphenyl)phthalazine-N,C 2 '](2,2,6,6-tetramethyl-3,5-heptanedione) iridium, with 1-methyl-4-(2',3'-dimethylphenyl)phthalazine as Cyclometallic ligands, the structural formula is as follows:
[0092]
[0093] Wherein, the structural formula of the ring metal ligand is:
[0094]
[0095] The preparation method of the metal iridium complex of the above-mentioned red light organic electrophosphorescent material comprises the following steps:
[0096] (1) Provide compound A and compound B2 represented by the following structural formula respectively:
[0097]
[0098] Among them, compound A is 1-methyl-4-chlorophthalazine, and B2 is 2,3-dimethylphenylboronic acid.
[0099] (2) Synthesis of cyclometal ligand 1-methyl-4-(2',3'-dimethylphenyl)phthalazine
[0100]
[0101] Under nitrogen protection, 0.72g (4.0mmol) 1-methyl-4-chlorophthalazine, 0.6g (4mmol) 2,3-m...
Embodiment 3
[0122] A red-light organic electrophosphorescent material metal iridium complex bis[1-methyl-4-(3',6'-dimethylphenyl)phthalazine-N,C 2 '](2,2,6,6-tetramethyl-3,5-heptanedione)iridium, with 1-methyl-4-(2',4'-dimethylphenyl)phthalazine as Cyclometallic ligands, the structural formula is as follows:
[0123]
[0124] Wherein, the structural formula of the ring metal ligand is
[0125]
[0126] The preparation method of the metal iridium complex of the above-mentioned red light organic electrophosphorescent material comprises the following steps:
[0127] (1) Provide compound A and compound B3 represented by the following structural formula respectively:
[0128]
[0129] Among them, compound A is 1-methyl-4-chlorophthalazine, and B3 is 2,5-dimethylphenylboronic acid.
[0130] (2) Synthesis of cyclometal ligand 1-methyl-4-(2',5'-diylphenyl)phthalazine
[0131]
[0132] Under nitrogen protection, 0.72g (4.0mmol) 1-methyl-4-chlorophthalazine, 0.72g (4.8mmol) 2,5-dime...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com