Symmetric short-sequence antimicrobial peptide analogues and application thereof
An antibacterial peptide and analog technology, applied in the field of biochemistry, can solve the problems of long sequence, high manufacturing cost, complex design, etc., and achieve the effects of low drug resistance, low manufacturing cost, and simple design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
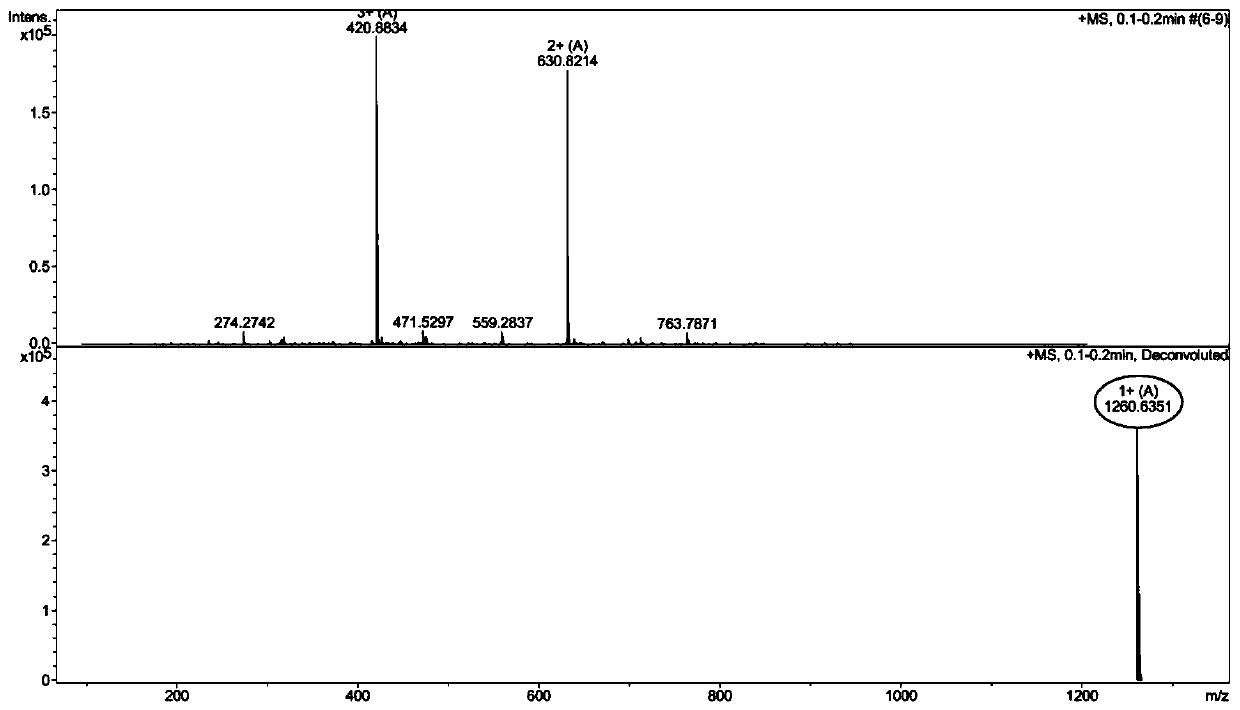
[0047] Example 1: Synthesis of WRW and its in vitro antibacterial activity and hemolytic toxicity research
[0048] (1) Synthesis of WRW
[0049] ①Activation and pretreatment of resin
[0050] Accurately weigh 0.69g of MBHA resin (substitution value 0.44mmol / g) and place it in a synthesizer. After swelling with DCM solution for 30min, the ninhydrin colorimetry test shows that the resin is colorless and transparent, indicating that the resin is normal.
[0051] ② Synthesis of WRW-resin
[0052] The normal MBHA resin of the above-mentioned inspection was sloughed off the Fmoc protecting group through the DMF solution containing 20% piperidine by volume fraction, and the ninhydrin chromogenic method was tested, and the resin was blue-purple, indicating that the Fmoc protecting group had been removed; the Fmoc-Trp ( Boc)-OH (390mg), HOBT (123mg), HBTU (342mg), and DIEA (0.3ml) were dissolved in 8ml DMF and mixed evenly, and mixed with the above-mentioned MBHA resin whose Fmoc ...
Embodiment 2
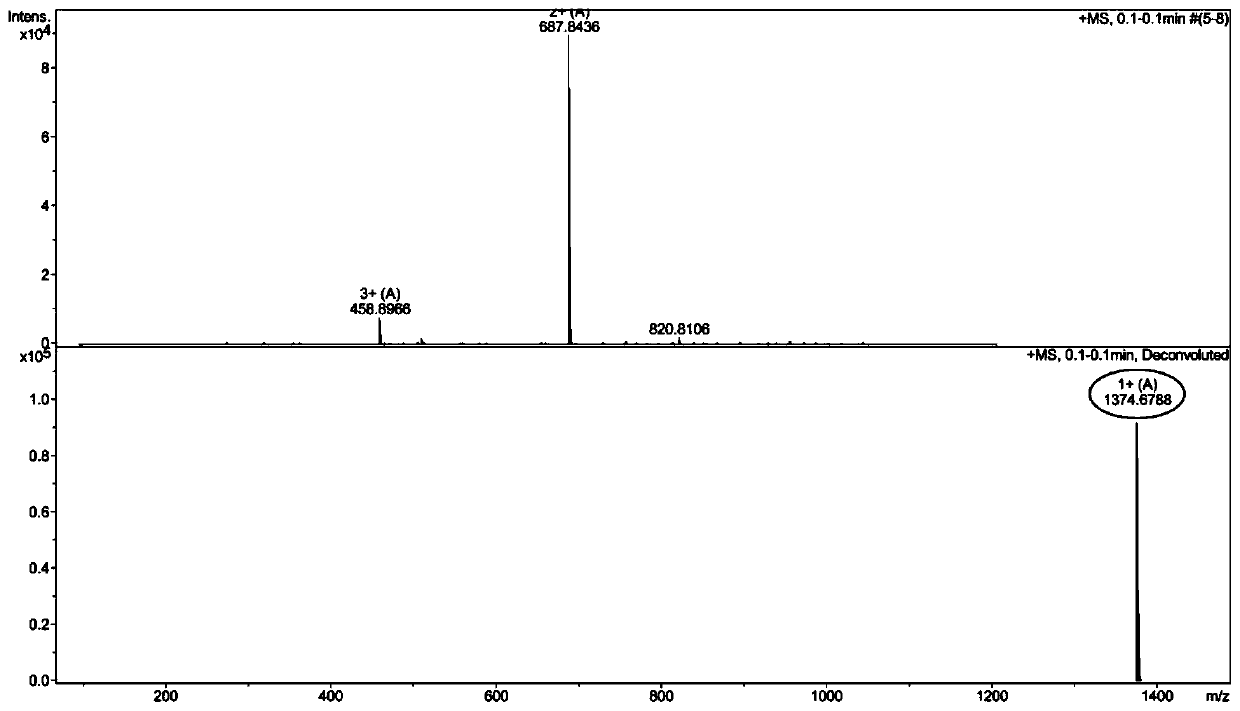
[0063] Example 2: Synthesis of GWRW and its in vitro antibacterial activity and hemolytic toxicity research
[0064] (1) Synthesis of GWRW
[0065] ①Activation and pretreatment of resin
[0066] With embodiment 1.
[0067] ② Synthesis of GWRW-resin
[0068] For the above-mentioned normal MBHA resin, the Fmoc protecting group was removed by the DMF solution containing 20% piperidine by volume fraction, and the ninhydrin chromogenic method was used to detect that the resin was blue-purple, indicating that the Fmoc protecting group had been removed; the Fmoc-Gly- OH (267mg), HOBT (123mg), HBTU (342mg), and DIEA (0.3ml) were dissolved in 8ml DMF and mixed evenly, and mixed with the above-mentioned MBHA resin whose Fmoc protecting group was removed, and condensed for 1 hour; ninhydrin developed color If the resin is colorless and transparent, it indicates that the condensation reaction is successful and Fmoc-Gly-resin is obtained; the method is the same as above, and the follo...
Embodiment 3
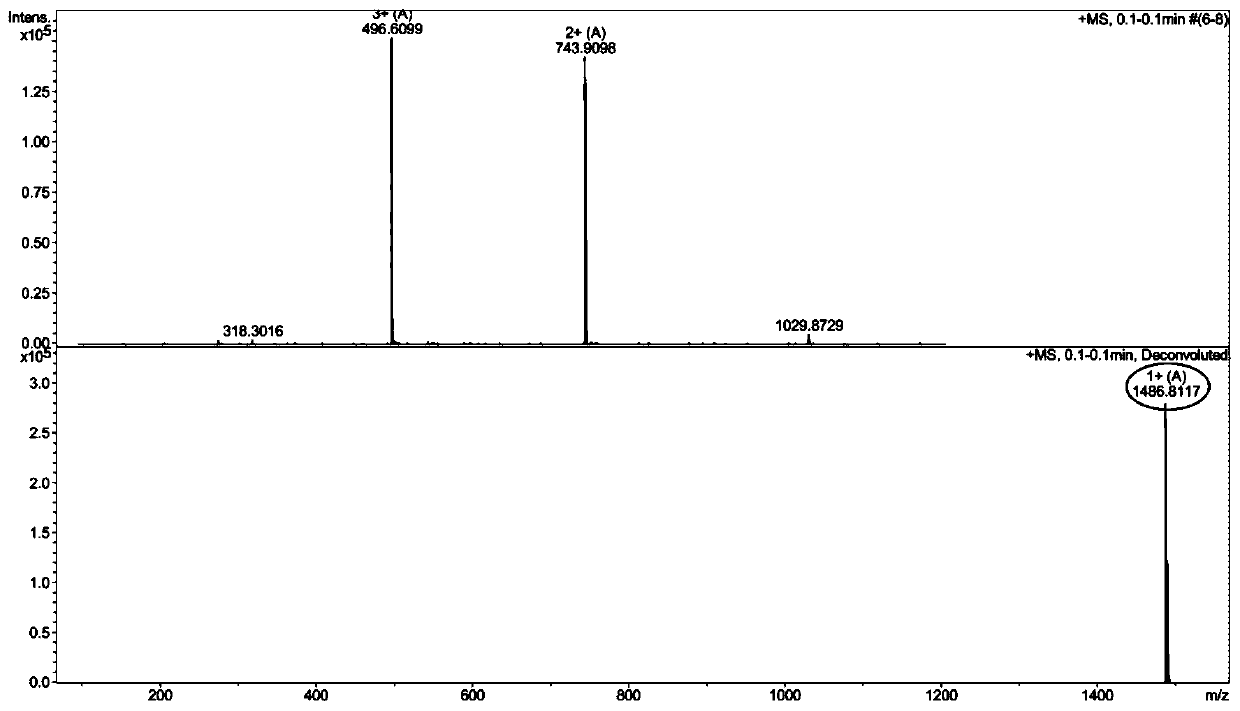
[0079] Example 3: Synthesis of IWRW and its in vitro antibacterial activity and hemolytic toxicity research
[0080] (1) Synthesis of IWRW
[0081] ①Activation and pretreatment of resin
[0082] With embodiment 1.
[0083] ②Synthesis of IWRW-resin
[0084] The normal MBHA resin of the above-mentioned inspection was removed the Fmoc protecting group through the DMF solution containing 20% piperidine by volume fraction, and the ninhydrin chromogenic method was tested, and the resin was blue-purple, indicating that the Fmoc protecting group had been removed; the Fmoc-Ile- OH (399mg), HOBT (123mg), HBTU (342mg), DIEA (0.3ml) were dissolved in 8ml DMF and mixed evenly, and mixed with the above-mentioned MBHA resin from which the Fmoc protecting group was removed, and the condensation reaction was carried out for 1 hour; ninhydrin developed color Method test, the resin is colorless and transparent, it shows that the condensation reaction is successful, and Fmoc-Ile-resin is obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com