Novel recombinant bee venom polypeptide as well as preparation method and application thereof
A new type of bee venom technology, applied in the field of novel recombinant bee venom polypeptide and its preparation, can solve the problems of restricting melittin, malignant transformation of cells, affecting the efficacy of melittin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: A specific preparation method of a novel recombinant bee venom polypeptide comprises the following steps:
[0030] 1. Synthesize the nucleotide sequence encoded by the novel recombinant bee venom polypeptide gene: react the nucleotide with the protected active group pre-connected on the solid phase carrier CPG with trichloroacetic acid to remove the protection of its 5'-hydroxyl group Group DMT, obtain free 5'-hydroxyl, synthesize DNA raw material phosphoramidite to protect nucleic acid monomer, mix with activator tetrazolium, obtain nucleoside phosphorous acid activated intermediate, activate 3' end, 5'- The hydroxyl group is still protected by DMT, and it undergoes a condensation reaction with the free 5'-hydroxyl group in the solution, followed by a capping reaction. In the condensation reaction, there may be very few 5'-hydroxyl groups that do not participate in the reaction. Use acetic anhydride and 1-methylimidazole Terminate the subsequent reaction,...
Embodiment 2
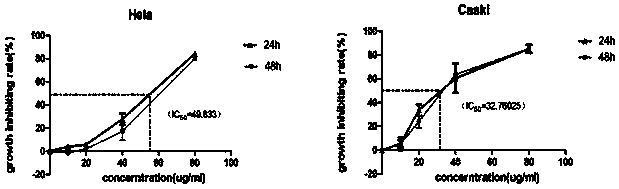
[0034] Example 2: Effect of novel recombinant bee venom polypeptide on cervical cancer cell lines and expression of HPV16 / 18E6 and E7
[0035] (1) Growth inhibition test of novel recombinant bee venom polypeptide cervical cancer cell line
[0036] a. Hela (HPV18 positive) and Caski (HPV16 positive) cells in the logarithmic growth phase were selected and digested with trypsin to make a cell suspension, counted on a cell counting plate, and diluted to a density of 4*104 / ml. After fully mixing the diluted cells, inoculate them in a well plate, and add 100ul to each well (add along the side wall, do not shake).
[0037] b. There are four concentration gradient groups of 0ug / ml, 20ug / ml, 40ug / ml, and 80ug / ml, and three replicate holes are set for each concentration. After the cells adhered to the wall for 12 hours, different concentrations of drugs were added to act for 24 hours and 48 hours. Discard the medium containing the drug, and add new medium containing 10% CCK-8 solution...
Embodiment 3
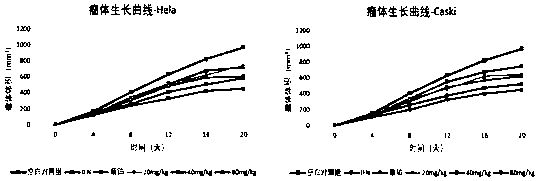
[0056] Example 3: Novel recombinant mee venom polypeptide inhibits cervical cancer and HPV16 / 18E6, E7 expression in vivo test on tumor-bearing nude mice
[0057] (1) Establishment and grouping of tumor-bearing nude mouse models
[0058] a. Balb / c-nu nude mice, female, age 6-8 weeks, body weight 18-22g, raised in SPF environment, 60 in total. HeLa and Caski cells in the logarithmic growth phase were digested with 0.25% trypsin, centrifuged and washed twice with serum-free culture medium, and the cell concentration was adjusted to 10^7 cells / ml with serum-free culture medium. Under sterile conditions, 0.2 ml of the cell suspension was extracted with a syringe with a No. 6 needle and inoculated subcutaneously in the right axilla of nude mice sterilized with 75% alcohol.
[0059] b. About 4 days after the inoculation, nodules appeared under the skin of the nude mice or until the tumor tissue grew to about 150mm 3 , perform random grouping. Volume V = long diameter * short diame...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com