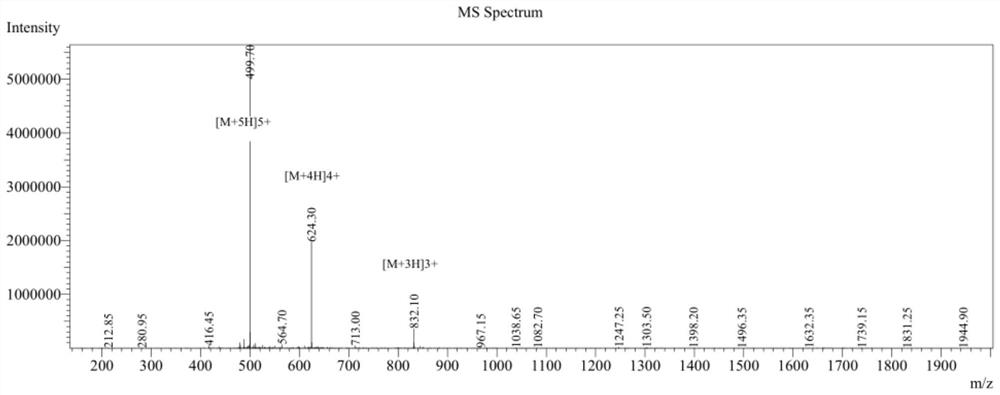
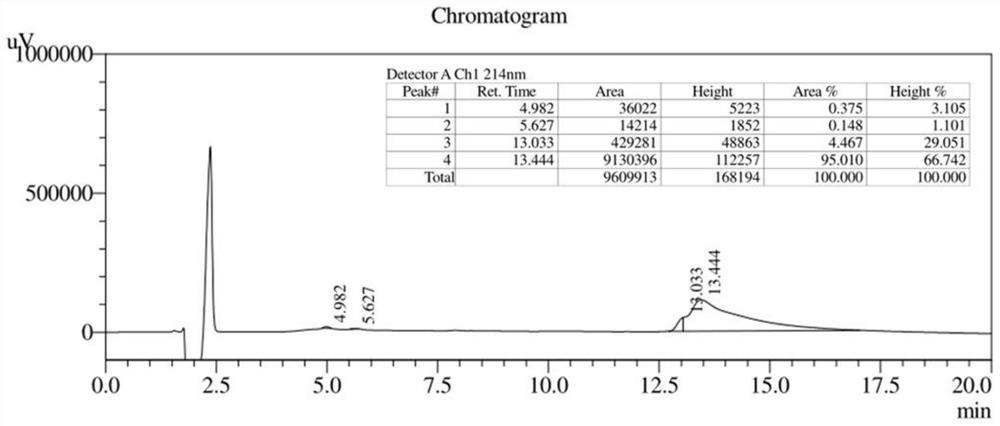
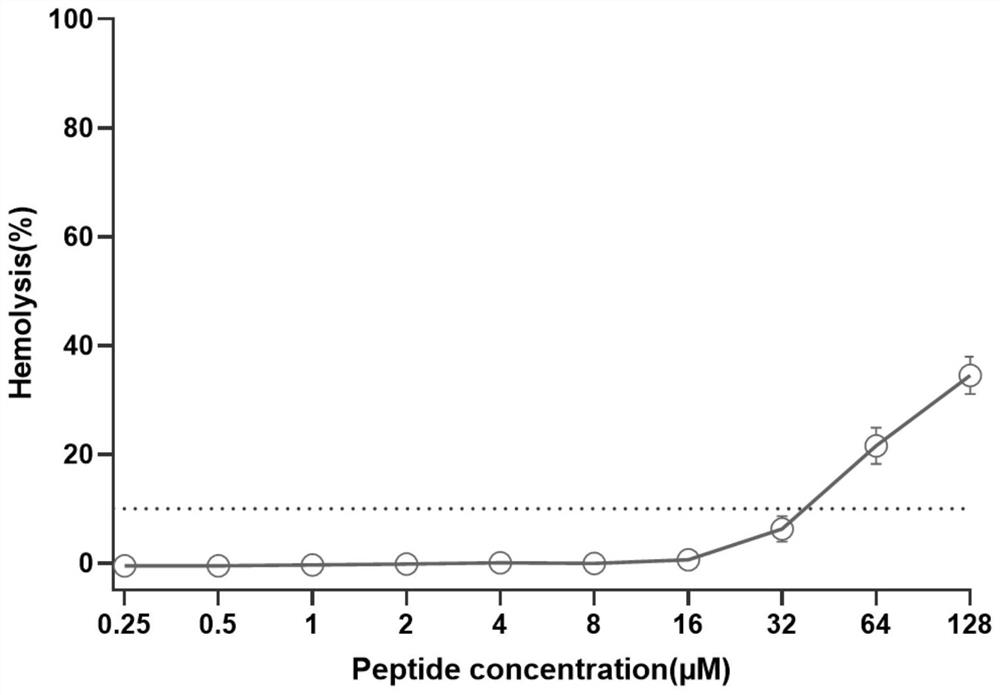
Anti-enzymolysis branched antibacterial peptide Pal-CRKP as well as preparation method and application thereof
An anti-enzymolysis, branched technology, applied in the biological field, can solve the problems of high sensitivity, short half-life, etc., and achieve the effects of high-efficiency inhibition, improved stability, and low hemolytic toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Design of branched antimicrobial peptides:
[0021] The amino acid sequence of the branched antimicrobial peptide Pal-CRKP is:
[0022] C 16 -Gly Gly Gly Lys (Cys Arg Lys Pro Cys Arg Lys Pro)Cys Arg Lys ProCys Arg Lys Pro
[0023] This design takes advantage of the characteristics of the lysine side chain, uses Lysine Lys as the site for linking n-hexadecanoic acid and anti-enzymatic hydrolysis sequences, uses the carboxyl group of Lysine Lys and the -NH on R group 2 Both link two anti-digestion sequences: Cys Arg Lys ProCys Arg Lys Pro, further use the flexible amino acid linker GGG to link n-hexadecanoic acid and lysine Lys, forming a branch with two anti-digestion sequence branches Antibacterial peptides and anti-enzymolysis sequences are based on the principle of reasonably avoiding the restriction site, avoiding restriction by trypsin, chymotrypsin and other proteases, and placing cysteine Cys and lysine Lys on the N of arginine Arg respectively. end and C-ter...
Embodiment 2
[0030] The above-mentioned dendritic antibacterial peptide is synthesized using a peptide synthesizer, the method is a solid-phase chemical synthesis method, and the specific steps are:
[0031] Step 1. The preparation of the polypeptide backbone is carried out one by one from the C-terminus to the N-terminus, and is completed by a polypeptide synthesizer. First, Fmoc-X (X is the first amino acid at the C-terminus of each antimicrobial peptide) was inserted into Wang resin, and then the Fmoc group was removed to obtain X-Wang resin; then Fmoc-Y-Trt-OH (9 -Fluorenemethoxycarboxy-trimethyl-Y, Y is the second amino acid at the C-terminus of each antibacterial peptide); follow this procedure to synthesize from the C-terminus to the N-terminus until the synthesis is completed, and obtain the side with the Fmoc group removed. Chain-protected resins;
[0032] Step 2. Use hydrazine hydrate to remove the Dde protecting group on the side chain of Fmoc-Lys(Dde)-OH, and repeat step 1 to ...
Embodiment 3
[0037] The antibacterial activity and hemolytic activity of the prepared anti-enzymatic branched antimicrobial peptide Pal-CRKP were detected in vitro;
[0038] 1. Determination of antibacterial activity: to measure the minimum inhibitory concentration (MIC) of the peptide is to inoculate the bacteria in Mueller-Hinton broth (MHB) at 37°C overnight and then transfer to a new medium until the logarithmic growth phase. Dilute the bacterial culture to 1×10 5 CFU / ml, 50 μl of BSA (pH=6.0) containing different concentrations of peptides were added to the above 96-well plates, and the final peptide concentrations in the 96-well plates ranged from 0.125 to 64 μM. Peptide solution and bacterial culture solution were mixed in equal volume 1:1 in a 96-well plate, cultured for 18 hours, and the optical density (OD) of the mixture was measured at 492 nm with a microplate reader (Tecan GENios F129004, Austria) to determine the minimum inhibitory concentration. , and the test results are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com