Antibacterial hexapeptides and their derivatives and applications
A technology of peptide derivatives and drugs, applied in the direction of antibacterial drugs, peptides, peptide/protein components, etc., can solve the problems of poor enzyme resistance and stability, cytolytic toxicity, easy degradation, etc., and achieve low hemolytic toxicity and broad-spectrum killing activity. , the effect of strong antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
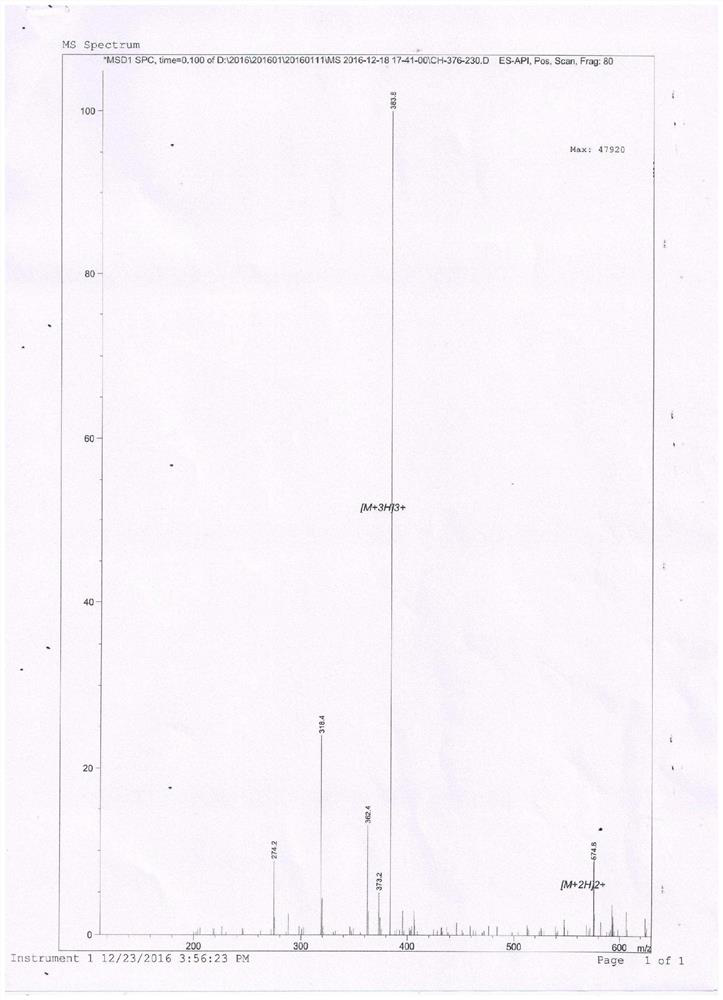
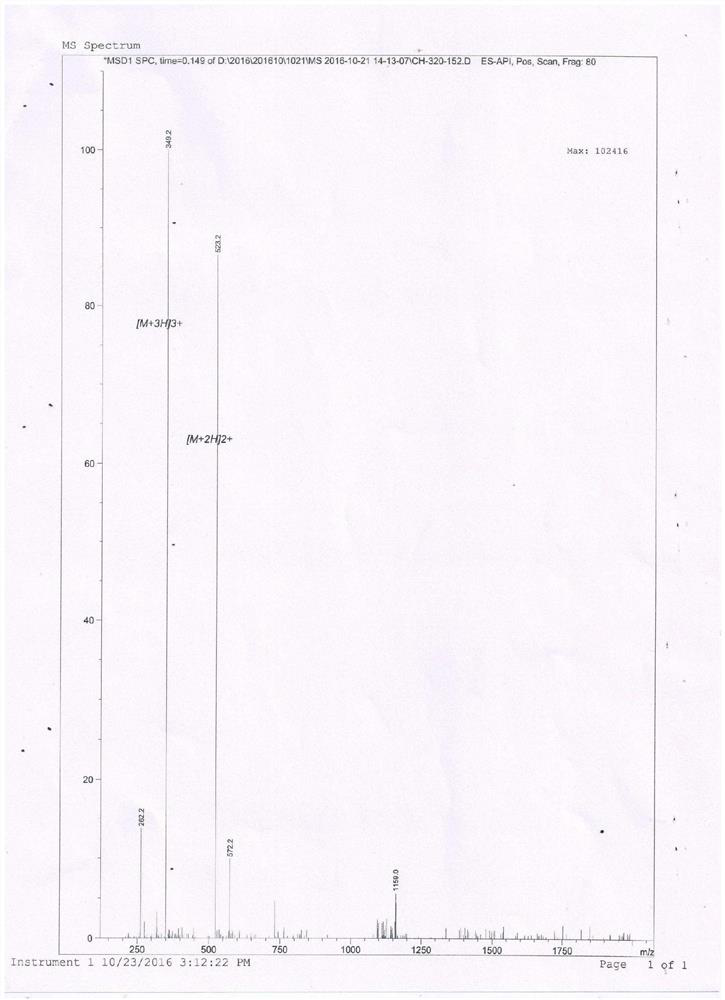
[0020] Chemically synthesized antimicrobial peptide a and antimicrobial peptide b and control antimicrobial peptide Lactoferricin B4-9.
[0021] Antimicrobial Peptide a:RRWWRW(Arg-Arg-Trp-Trp-Arg-Trp)
[0022] Antimicrobial peptide b: RRWWRW-PEA (Arg-Arg-Trp-Trp-Arg-Trp-β-phenethylamine)
[0023] 1. Preparation of antimicrobial peptide b (Arg-Arg-Trp-Trp-Arg-Trp-β-phenethylamine):
[0024] a. After washing the resin with 4 times the amount of DMF and drying it completely, add 10 mL of 20% (piperidine: DMF, mass ratio) mixed solution of piperidine and DMF, shake for 1 min and dry, then add 10 mL of 20% (piperidine: DMF) , mass ratio) piperidine, DMF mixed solution shake for 30min; dry the reaction vessel and wash the resin with 4 times the amount of DMF to ensure that there is no piperidine residue, check the resin particles with ninhydrin should be blue.
[0025] b. Add Fmoc protected amino acid (1mmol), 2.1mL 0.45M HBTM / HOBT (1mmol), 248uL DIEA (2mmol) solution to the resin...
Embodiment 2
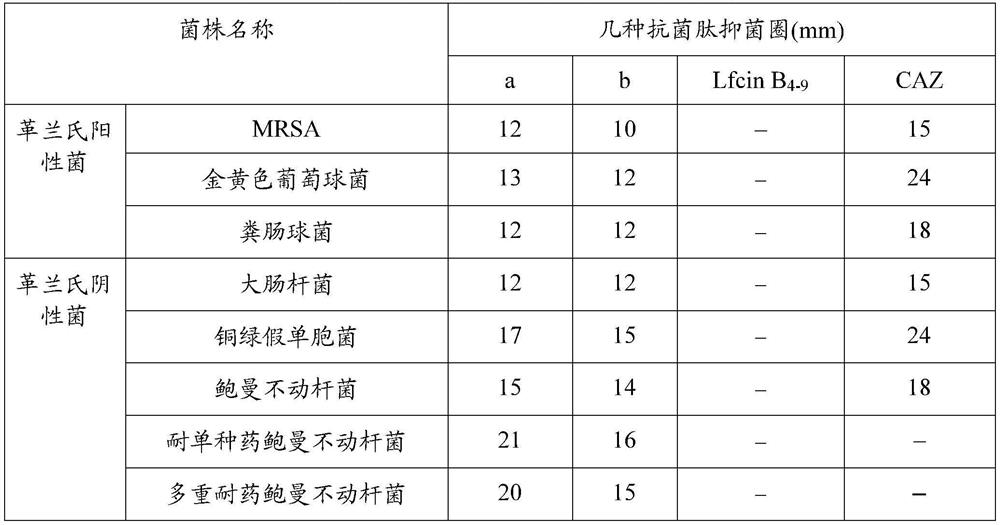
[0031] Antibacterial activity detection of antimicrobial peptides
[0032] The various standard strains used below were purchased from the Guangdong Provincial Microbial Culture Collection Center, and the drug-resistant bacteria were provided by the Third Military Medical University.
[0033] The antibacterial activities of synthetic antimicrobial peptide b and antimicrobial peptide a were detected by agar plate diffusion method, and the natural antimicrobial peptide Lfcin B 4-9 As a control, to evaluate the bactericidal activity of antimicrobial peptide b and antimicrobial peptide a in the present invention.
[0034] The antibacterial activity of antimicrobial peptides was determined by the following steps:
[0035] a. Bacterial recovery: Use an inoculation loop to pick appropriate amounts of Escherichia coli, Staphylococcus aureus, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterococcus faecalis, MRSA, and single drug-resistant Acinetobacter baumannii (for Ceftazidim...
Embodiment 3
[0043] Example 3 Detection of antibacterial activity of synthetic antimicrobial peptides
[0044] The various standard strains used below were purchased from the Guangdong Provincial Microbial Culture Collection Center, and the drug-resistant bacteria were provided by the Third Military Medical University.
[0045] The bactericidal activity of synthetic antimicrobial peptides was detected by 96-well plate method, and the natural antimicrobial peptide Lfcin B was used to detect the bactericidal activity. 4-9 As a control, to evaluate the antibacterial activity of antimicrobial peptides a and b.
[0046] The antibacterial activity of antimicrobial peptides was determined according to the following steps:
[0047] a. Sterilize Escherichia coli, Staphylococcus aureus, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterococcus faecalis, MRSA, single-drug-resistant Acinetobacter baumannii, and multidrug-resistant Acinetobacter baumannii Culture on NA medium plate overnight, pic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com