Anti-cancer drug, anti-cancer drug composition, and preparation method and application thereof
A technology of anticancer drugs and compositions, applied in the field of biomedicine, can solve the problems of poor selectivity, large toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
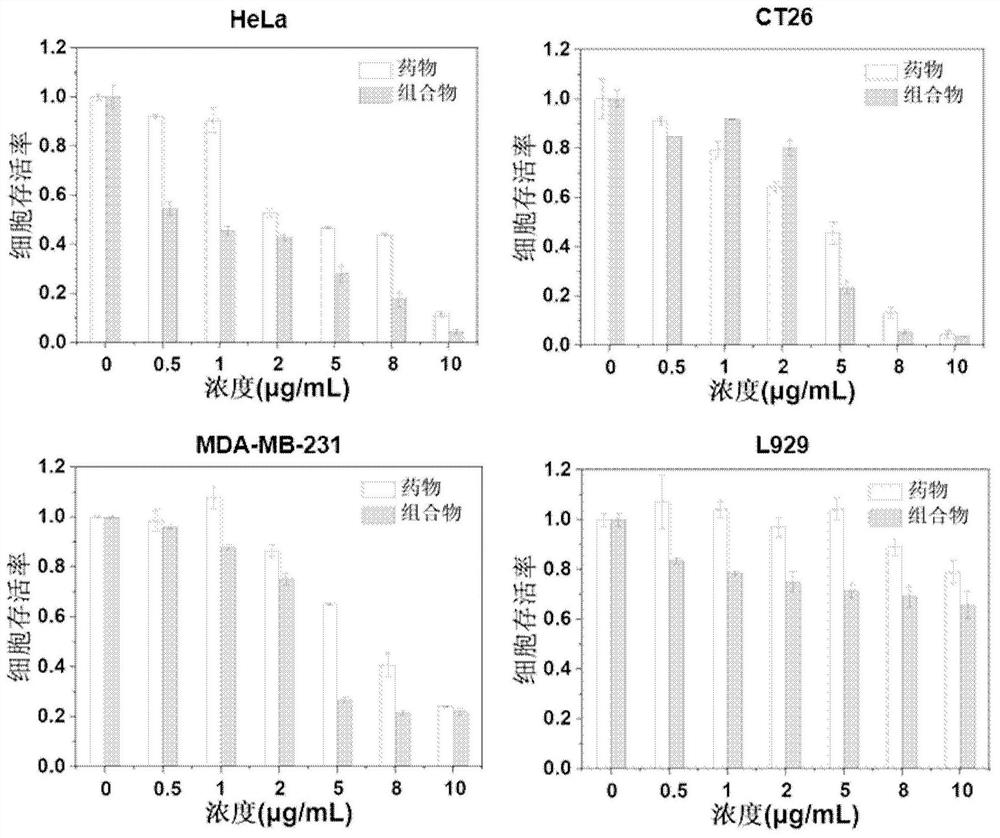
[0044] In vitro anticancer evaluation of 2-amino-5-[(5-nitro-2-thiazolyl)thio]-1,3,4-thiadiazole and its combination with phenylboronic acid polymer
[0045] MTT method was used to test the anti-cancer effect in vitro: take cancer cells (HeLa) and add them to 96-well plates, incubate in the incubator for 24 hours, add 100 μL of cell solution to each well, and inoculate 6×10 cells per well. 3 up to 8×10 3 , and the number of inoculations per well was approximately the same. 2-Amino-5-[(5-nitro-2-thiazolyl)thio]-1,3,4-thiadiazole (Formula I) and the composition are configured to a concentration of 0.5 mg / mL, where The solvent used for the structure of the coordination interaction is DMF, wherein in the anticancer drug composition 2-amino-5-[(5-nitro-2-thiazolyl) thio]-1,3,4-thiadiazole and The ratio of the amount of phenylboronic acid in the phenylboronic acid polymer is 1:1, the degree of polymerization of the segment where the phenylboronic acid is located in the phenylboron...
Embodiment 2
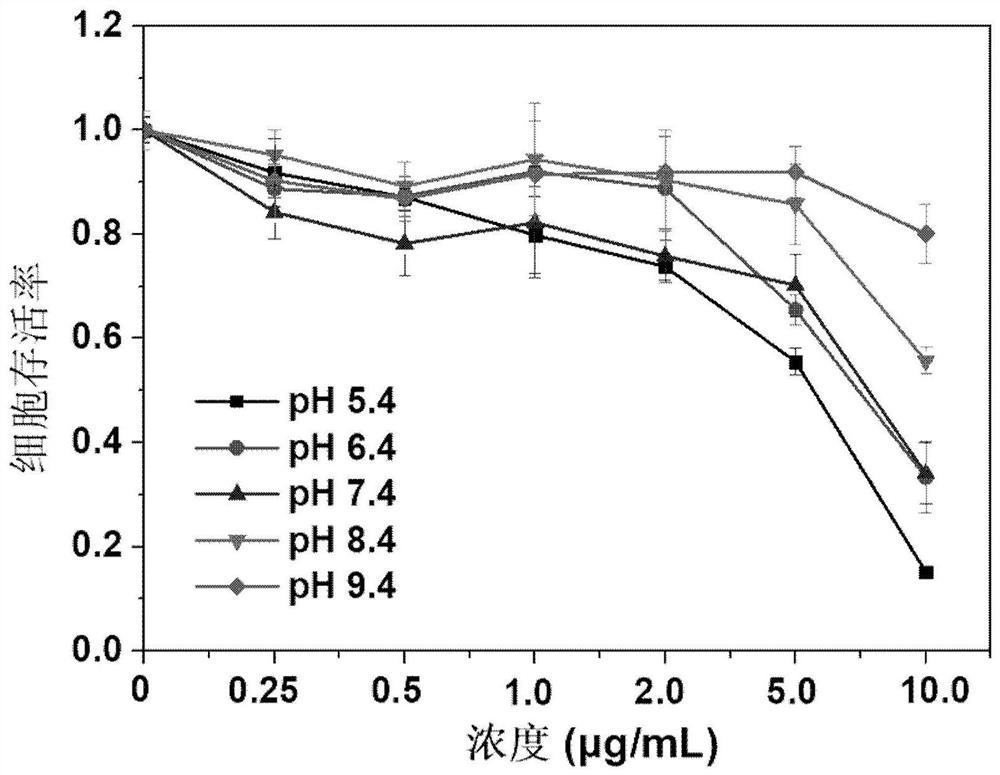
[0047] Correlation Evaluation of Proton Motive Potential of 2-amino-5-[(5-nitro-2-thiazolyl)thio]-1,3,4-thiadiazole
[0048] The proton kinetic potential related experiments were evaluated by the MTT method. Take 5 centrifuge tubes, add 10mL medium with serum in it, adjust the pH value with 1M hydrochloric acid and 1M sodium hydroxide, adjust to 5.4, 6.4, 7.4, 8.4, 9.4 respectively for use. Take cancer cells (HeLa) and add them to 96-well plates, incubate in the incubator for 24 hours, add 100 μL of cell solution to each well, and inoculate 6×10 cells per well. 3 up to 8×10 3 , and the number of inoculations per well was approximately the same. 2-Amino-5-[(5-nitro-2-thiazolyl)thio]-1,3,4-thiadiazole (Formula I) was formulated at a concentration of 0.5 mg / mL. Take culture media with different pH values, and prepare media containing different concentrations of anticancer drugs. The concentration of anticancer drugs is 0.25 μg / mL to 10 μg / mL. Cancer drug medium, incubated in ...
Embodiment 3
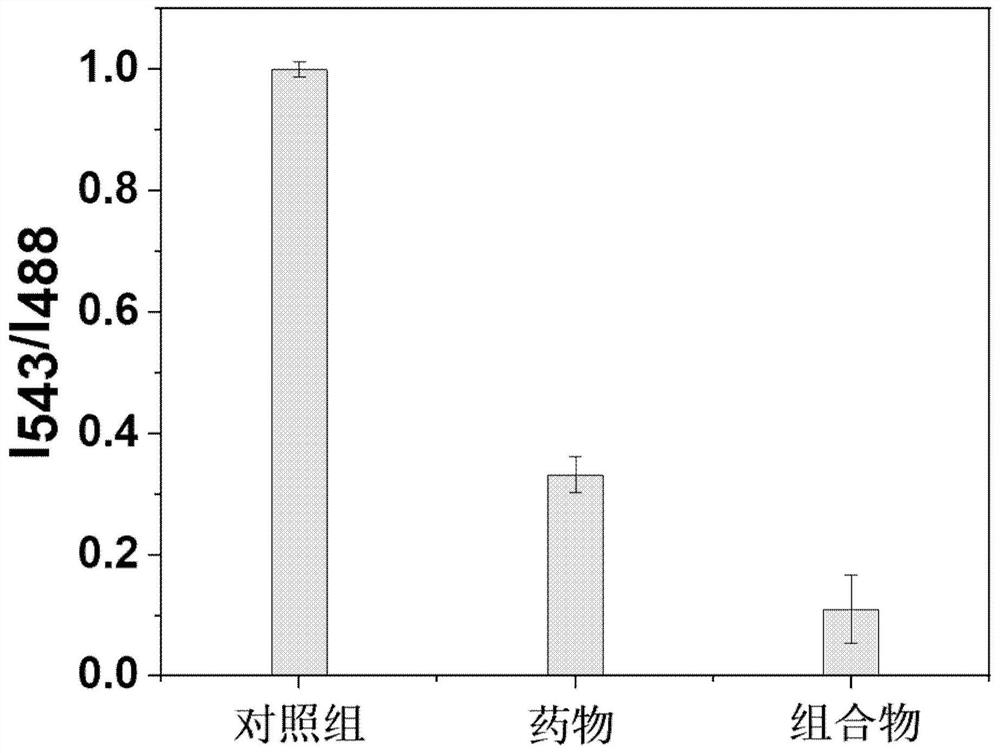
[0050] Evaluation of membrane potential change of 2-amino-5-[(5-nitro-2-thiazolyl)thio]-1,3,4-thiadiazole and its composition
[0051] The mitochondrial membrane potential detection kit JC-1 was used to stain the cells and observe the color change with a fluorescence microscope to evaluate the membrane potential. The kit JC-1 was purchased from Shanghai Beyond Biotechnology Co., Ltd., model C2006. Take cancer cells (HeLa) and add them to 24-well plates, incubate in the incubator for 24 hours, add 400 μL of cell solution to each well, and inoculate 2×10 cells per well. 4 to 4×10 4 , and the number of inoculations per well was approximately the same. Aspirate the original medium, add the prepared 2-amino-5-[(5-nitro-2-thiazolyl)thio]-1,3,4-thiadiazole (Formula I) at a concentration of 10 μg / mL for culture medium and incubated for 4 h in the incubator. Take an appropriate amount of JC-1 (200X), and dilute JC-1 by adding 8 mL of ultrapure water to every 50 μL of JC-1 (200X). V...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com