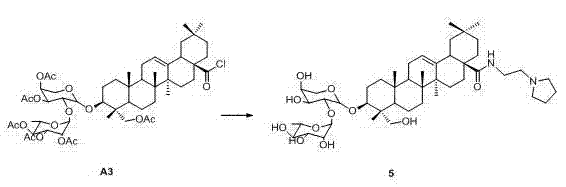
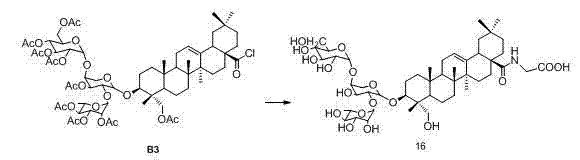
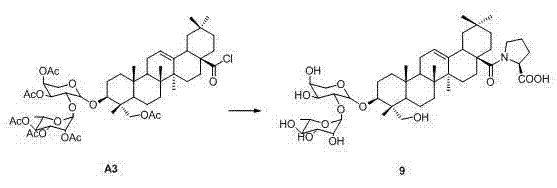
Novel alpha-hederin derivative and preparation method and use thereof
A technology of hedera saponins and derivatives, applied in the field of natural medicine, can solve the problems of unclear hemolytic mechanism, and achieve the effect of reducing hemolytic toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: 3-O-α-L-rhamnose-(1 → 2)-α-L-arabinose ivy saponin 28-carboxamide (1)
[0041] 1) Synthesis of intermediate A2
[0042]
[0043] The raw material α-hedera saponins (A1) (1.00 g, 1.36 mmol) was dissolved in anhydrous pyridine (10 mL), acetic anhydride (1.3 mL, 13.6 mmol) was added with stirring, and the mixture was stirred at room temperature for 4 hours. After the reaction is over, add 100ml ethyl acetate, adjust pH 4-5 with 10% HCl; wash the organic layer with saturated brine (50 mL×3), dry with anhydrous sodium sulfate, filter, concentrate under reduced pressure to remove the solvent, silica gel column layer After analysis (100 g, petroleum ether-ethyl acetate 2:1), intermediate A2 (white solid 1.30 g) was obtained with a yield of 95.0%. 1 H-NMR (500MHz, CDCl 3 ): δ 5.21(brs, 1H, H-12), 4.97(s, J = 5.0 Hz,1H, H-1''), 4.44(d, J =5.0 Hz, H-1'), 2.14, 2.11, 2.09, 2.05, 2.02, 1.97 (s each, 3H each, 7×CH 3 CO), 1.21(d, J = 5.0 Hz, 3H, H-6``), 1.11, 0.99, 0.95, 0.93...
Embodiment 2
[0051] Example 2: 3-O-α-L-rhamnose-(1 → 2)-α-L-arabinose ivy saponin 28-cyclopropanamide (2)
[0052]
[0053] Intermediate A3 (510.8 mg, 0.5 mmo) was dissolved in anhydrous dichloromethane (10 mL), and cyclopropylamine (44 mg, 0.75 mmol) and triethylamine (101.2 mg, 1.0 mmol) were added under ice cooling, and reacted at room temperature for 12 hours. After the reaction is complete, add 50 mL of dichloromethane, wash with saturated brine (50 mL×3), dry with anhydrous sodium sulfate, filter, and concentrate under reduced pressure to remove the solvent to obtain a white solid. The white solid was dissolved in anhydrous methanol (20 mL), sodium methoxide (20 mg) was added, and the mixture was stirred at room temperature for 12 h. After the reaction was completed, the solvent was removed under reduced pressure, and silica gel column chromatography (60 g, dichloromethane: methanol = 8:1) was used to obtain compound 2 (330.2 mg). The yield of the two steps was 81.2%.
[0054] White soli...
Embodiment 3
[0055] Example 3: 3-O-α-L-rhamnanopyranos-(1 → 2)-α-L-arabinose ivy saponin 28-morpholinocarboxamide (3)
[0056]
[0057] The preparation method of compound 3 is similar to that of Example 2, and the yield is 81.4%.
[0058] Compound 3, white solid, melting point 195-197°C. 1 H-NMR (500MHz, pyridine- d 6 ): δ 6.17(s, 1H, H-1''), 5.51(brs, 1H, H-12), 5.06(d, J = 5.0 Hz, H-1'), 3.68-3.86(m, 8H, H-morpholine) 1.73(3H, d, J = 5.0 Hz, H-6``), 1.31, 1.14, 1.10, 1.08, 1.00, 0.96( s each, 3Heach, 6×CH 3 ); 13 C-NMR(125MHz, pyridine- d 6 ): 38.8(C-1), 26.1(C-2), 82.0 (C-3), 44.2(C-4), 48.6(C-5), 18.8(C-6), 33.3 (C-7) , 39.8(C-8), 48.8(C-9), 37.1(C-10), 23.9 (C-11), 122.1(C-12), 145.4(C-13), 42.6(C-14), 28.3(C-15), 24.3(C-16), 46.8(C-17), 42.3(C-18), 46.6(C-19), 30.7(C-20), 34.3(C-21), 33.3 (C-22), 65.2(C-23), 13.5(C-24), 16.2(C-25), 17.4(C-26), 26.1(C-27), 170.7(C-28), 33.4( C-29), 23.9(C-30), 104.7(C-1'), 76.2(C-2'), 74.2(C-3'), 68.9(C-4'), 65.9(C-5') ), 101.9( C-1''), 72.4( C-2``), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com