Antibacterial pentapeptide derivative and application thereof
A peptide derivative and anti-bacterial technology, applied in the direction of anti-bacterial drugs, peptides, peptide/protein components, etc., can solve the problems of poor enzyme resistance stability, cytolytic toxicity, easy degradation, etc., and achieve low hemolytic toxicity and broad-spectrum killing The effect of activity, strong antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
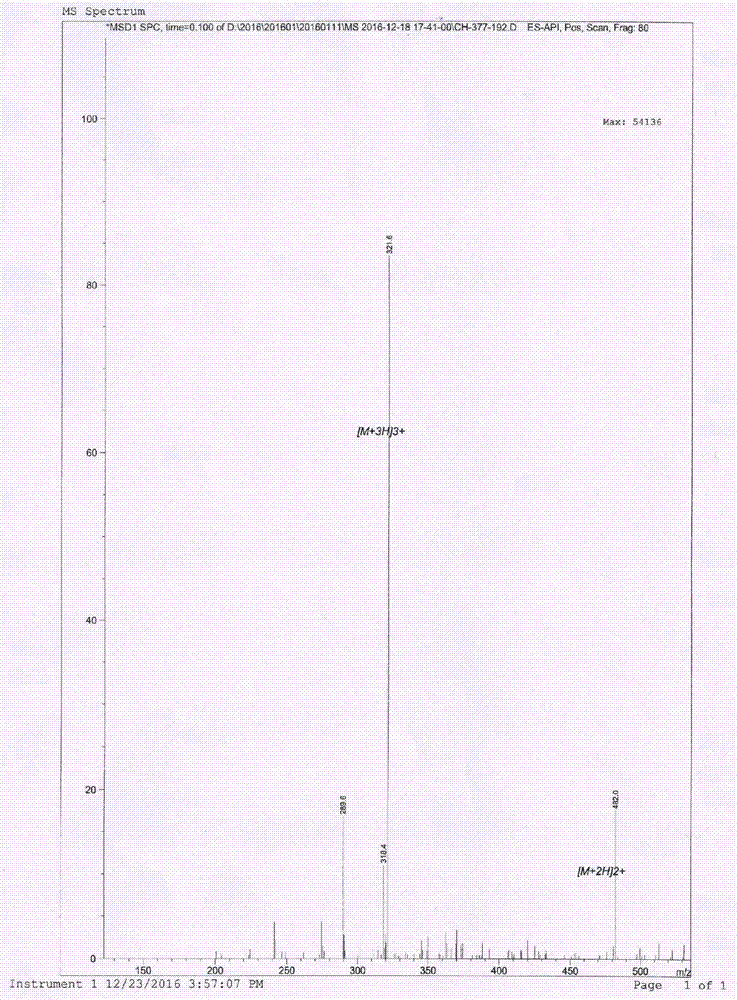
[0017] Chemical synthesis of antimicrobial peptide a and antimicrobial peptide b and control antimicrobial peptide Lactoferricin B 4-9 .
[0018] Antimicrobial peptide a: RRWWR-NH 2 (Arg-Arg-Trp-Trp-Arg-NH 2 )
[0019] Antimicrobial peptide b: RRWWR-PEA (Arg-Arg-Trp-Trp-Arg-β-phenylethylamine)
[0020] 1. Antimicrobial peptide a (Arg-Arg-Trp-Trp-Arg-NH 2 ) preparation:
[0021] The preparation is carried out one by one from the C-terminal to the N-terminal, and is automatically controlled by the synthesizer.
[0022] First, weigh 0.01mol of the resin combined with the first amino acid, namely Arg (purchased from Applied Biosystems, USA), and pack it into a column; then protect it with 20% piperidine dimethylformamide solution, wash it with dimethylformamide, 9-Fat methoxycarbonyl (Fmoc)-protected free amino acids were dissolved in carbodiimide (DCC), hydroxybenzotriazole (HOBt) / diisopropylethylamine (DIPEA), and the dissolved solution was placed on the column Cycle the ...
Embodiment 2
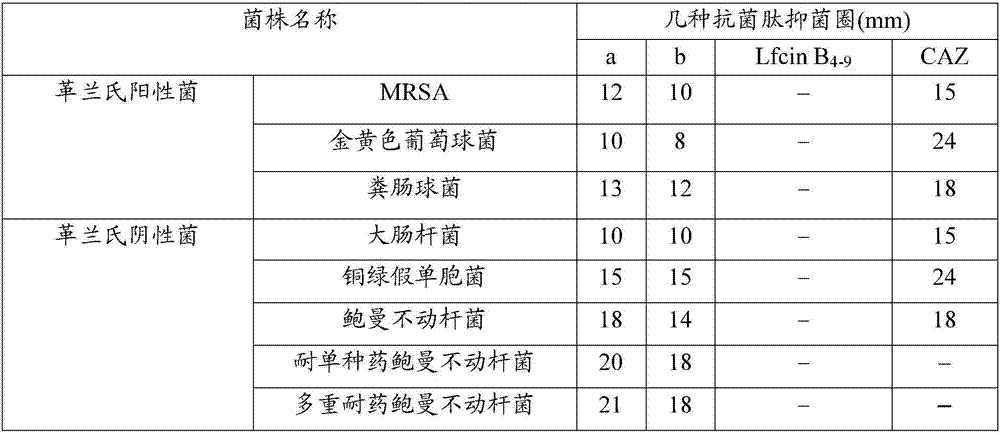
[0034] Detection of Antibacterial Activity of Antimicrobial Peptides
[0035] The various standard strains used below were purchased from the Guangdong Provincial Microbial Culture Collection Center and the drug-resistant bacteria were provided by the Third Military Medical University.
[0036] The antibacterial activity of synthetic antimicrobial peptide b was detected by agar plate diffusion method, and the antibacterial activity of natural antimicrobial peptide LfcinB 4-9 And antimicrobial peptide a as contrast, to evaluate the bactericidal activity of antimicrobial peptide b in the present invention.
[0037] Measure the antibacterial activity of antimicrobial peptides according to the following steps:
[0038] a. Strain recovery: Pick appropriate amount of Escherichia coli, Staphylococcus aureus, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterococcus faecalis, MRSA, single-drug resistant Acinetobacter baumannii (for Ceftazidime-resistant, the same below), and mul...
Embodiment 3
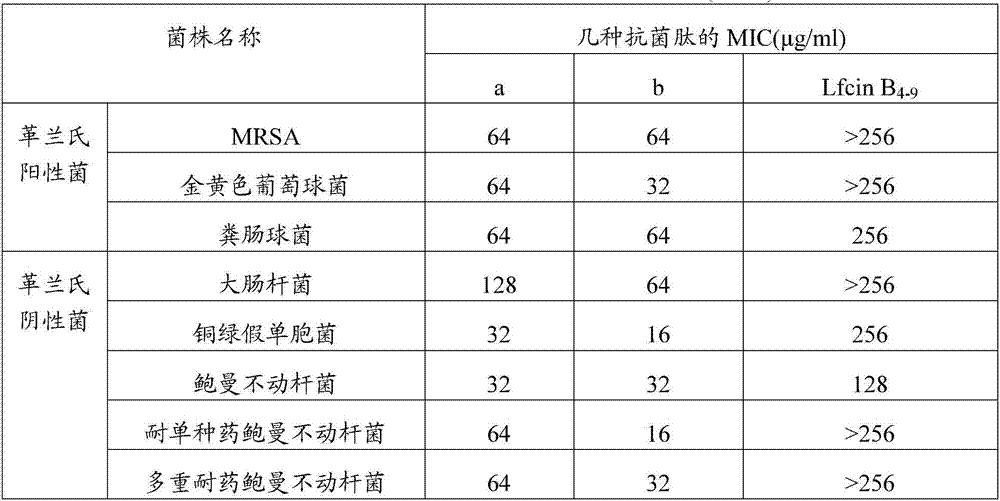
[0046] The bacteriostatic activity detection of embodiment 3 synthetic antimicrobial peptides
[0047] The various standard strains used below were purchased from the Guangdong Microbial Culture Collection Center, and the drug-resistant bacteria were provided by the Third Military Medical University.
[0048] The bactericidal activity of synthetic antimicrobial peptides was detected by 96-well plate method, and the natural antimicrobial peptide Lfcin B 4-9 As a control, to evaluate the antibacterial activity of antimicrobial peptides a and b.
[0049] Measure the antibacterial activity of antimicrobial peptides according to the following steps:
[0050] Experimental steps:
[0051] a. Escherichia coli, Staphylococcus aureus, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterococcus faecalis, MRSA, single-drug-resistant Acinetobacter baumannii, and multidrug-resistant Acinetobacter baumannii were sterilized Cultivate overnight on the NA medium plate, pick a single colony...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com