A kind of cyclic antimicrobial peptide analogue rich in positive charge and its application
A technology of antimicrobial peptides and analogs, applied in the field of biochemistry, can solve the problems of strong side effects and bacterial drug resistance, and achieve the effects of strong antibacterial activity, not easy to induce bacterial drug resistance, and high metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
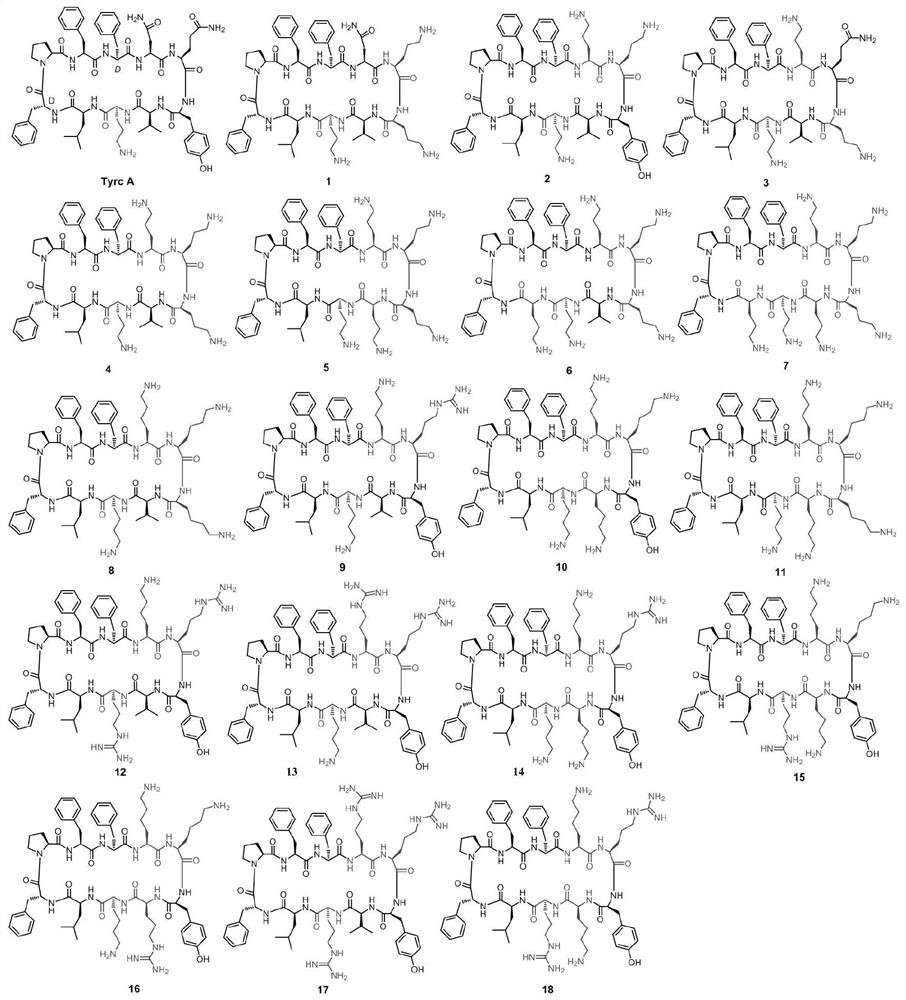
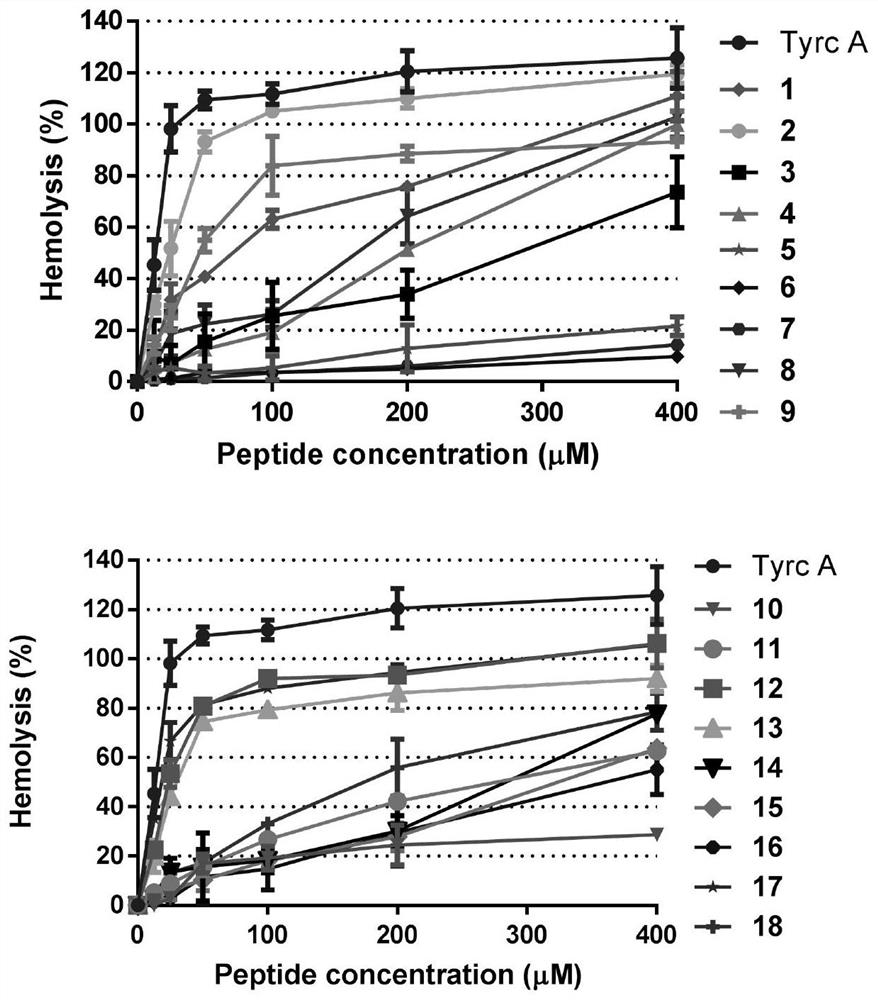
[0061] This example provides a positively charged cyclic antimicrobial peptide analogue, which is based on a derivative of the natural antimicrobial peptide gramicidin tyrosin A skeleton, which is obtained by introducing amino acids into its skeleton. Its structure The general formula is: cyclo-( D FPF D FXXXZXZ), wherein F=Phe, P=Pro, X=Gln, Orn, Lys, Arg, Tyr or Asn, Z=Val, Leu, Orn, Lys or Arg, and the left superscript D represents a D-type amino acid.
[0062] The structural formula of the cyclic antimicrobial peptide analog is as follows:
[0063] cycle -( D FPF D FNOOVOL), labeled as compound 1;
[0064] cycle -( D FPF D FOOYVOL), labeled as compound 2;
[0065] cycle -( D FPF D FOQOVOL), labeled as compound 3;
[0066] cycle -( D FPF D FOOOVOL), labeled as compound 4;
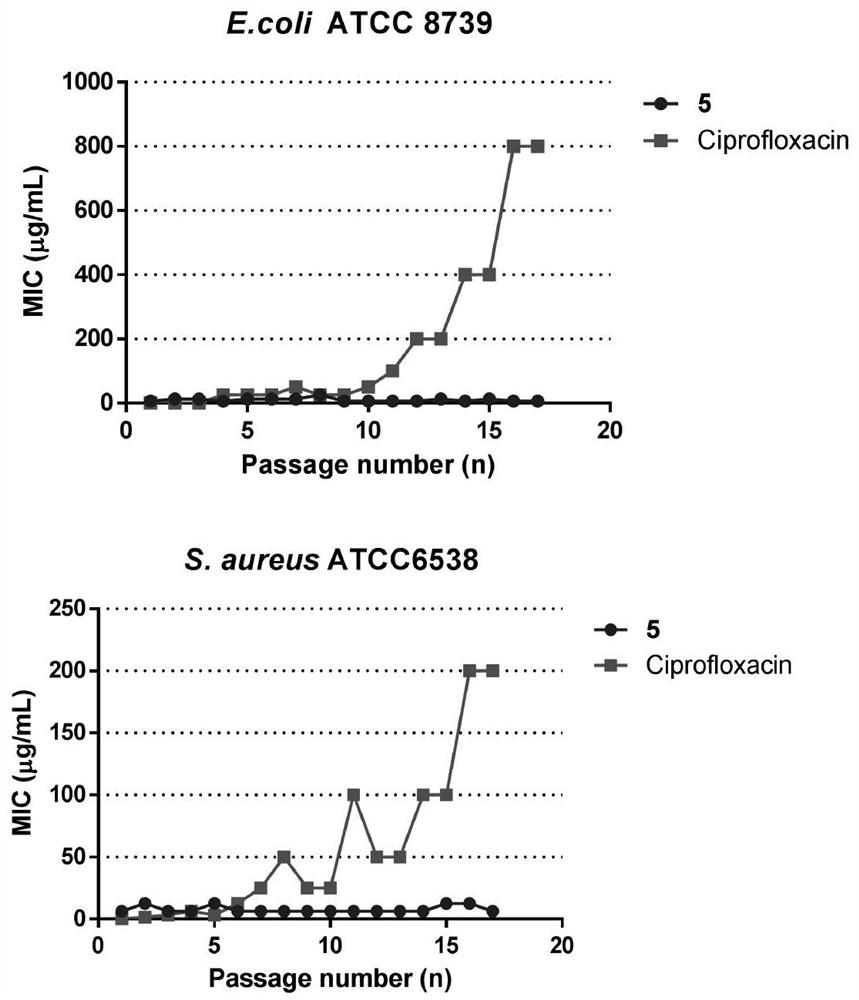
[0067] cyclo- ( D FPF D FOOOOOL), labeled as compound 5;
[0068] cycle -( D FPF D FOOOVOO), labeled as compound 6;
[0069] cycle -( D FPF D FOOOOOO), labeled as compound 7;...
Embodiment 2
[0086] This embodiment provides the synthesis method of compound 3, and the specific steps are:
[0087] (1) Swelling of the resin
[0088] Put 210mg of Fmoc-Pro-2-CTC resin (substitution value 0.476 mmol / g) in the peptide synthesis tube, and swell with DCM solution for 15 minutes;
[0089] (2) Synthesis of linear peptides
[0090] For the above-mentioned swollen Fmoc-Pro-2-CTC resin, the DMF solution containing 20% piperidine by volume was shaken twice for 15 minutes each time to remove the Fmoc protecting group; then washed twice with DMF and twice with MeOH, DCM washed 2 times, DMF washed 1 time, Fmoc- D Phe-OH (194 mg), HOBT (67.6 mg), HBTU (189 mg), and DIEA (0.25 mL) were dissolved and mixed in 5 mL of DMF, and mixed with the above-mentioned Fmoc-Pro-2-CTC resin deprotected from Fmoc Mix, shake and condense for 1 h to obtain Fmoc- D Phe-Pro-2-CTC; then washed again with DMF for 2 times, MeOH for 2 times, DCM for 2 times, and DMF for 1 time. The method is the same ...
Embodiment 3
[0102] This example provides the synthesis method of compound 4, the difference from Example 2 is that the synthesis steps of the linear polypeptide are:
[0103] For the above swollen Fmoc-Pro-2 CTC, oscillate twice with a DMF solution containing 20% piperidine by volume fraction, each time for 15 minutes, to remove the Fmoc protecting group; then wash twice with DMF, twice with MeOH, and then with DCM 2 times, washed 1 time with DMF, Fmoc- D Phe-OH (194 mg), HOBT (67.6 mg), HBTU (189 mg), and DIEA (0.25 mL) were dissolved in 5 mL DMF and mixed evenly, and mixed with the above-mentioned Fmoc-Pro-2 CTC resin that had removed the Fmoc protecting group Mix, shake and condense for 1 h to obtain Fmoc- D Phe-Pro-2 CTC; then washed again with DMF 2 times, MeOH 2 times, DCM 2 times, DMF 1 time. The method is the same as above, followed by condensation reaction of amino acids: Fmoc-Leu-OH (177 mg), Fmoc-Orn(Boc)-OH (227 mg), Fmoc-Val-OH (170mg), Fmoc-Orn(Boc)-OH ( 227 mg), Fmoc-O...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com