High-efficiency and low-toxicity pimaricin derivative as well as preparation method and application thereof
A high-efficiency and low-toxicity technology of pimaricin, applied in the field of high-efficiency and low-toxic pimaricin derivatives and its preparation, can solve the problems of low pharmacological properties and limiting the application value of pimaricin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
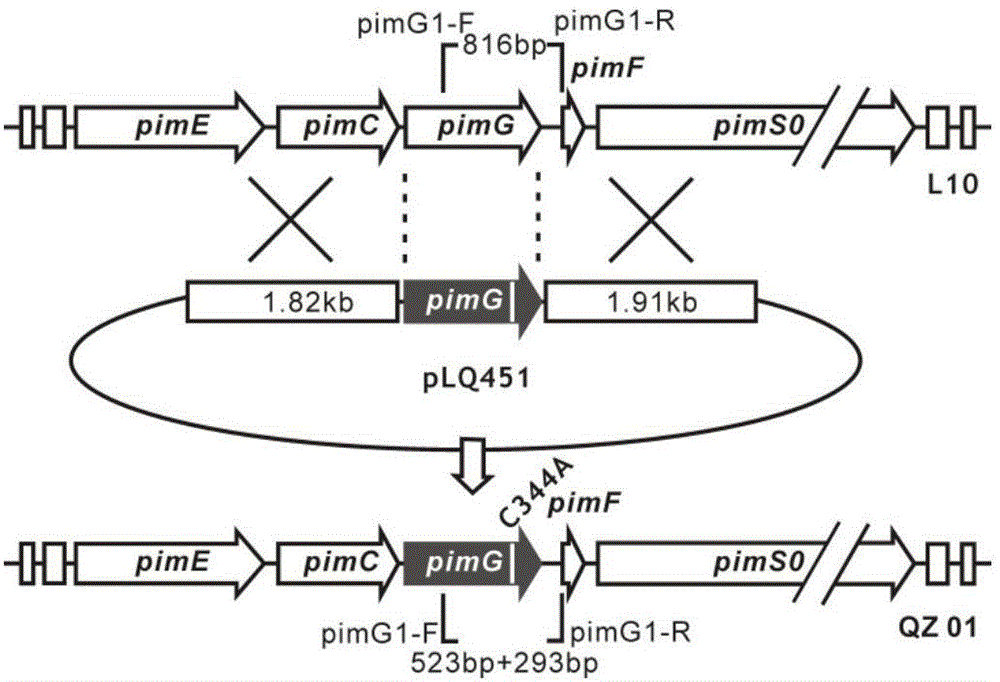
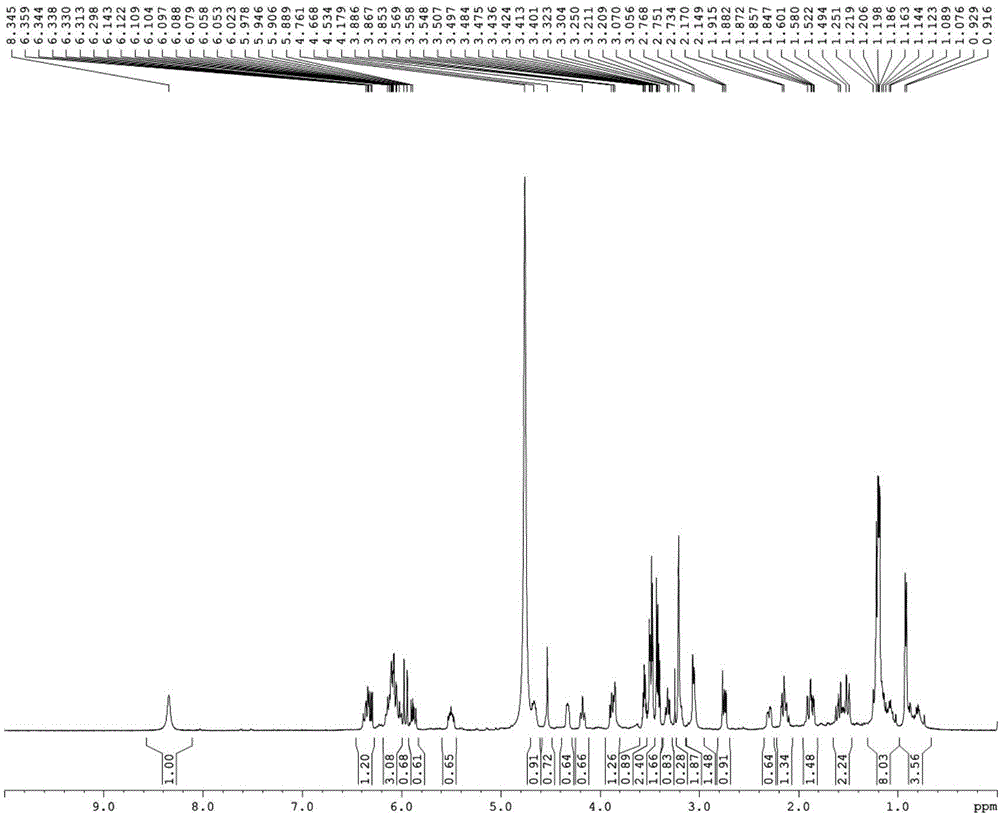
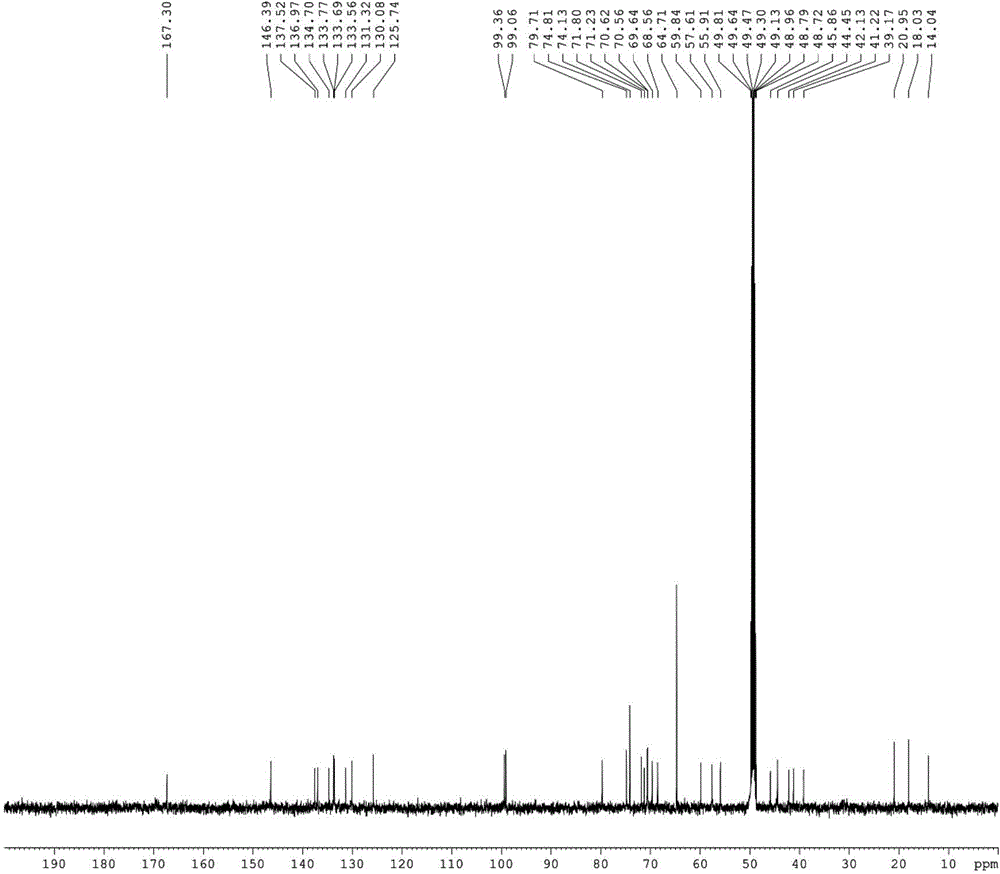
Image
Examples
Embodiment 1
[0031] Prepare 500ml seed medium and 5L fermentation medium according to the seed medium and fermentation culture formula, and divide them into 250ml three-sided flasks, 50ml each, sterilize at 115℃ for 30min, insert the spores of mutant QZ01 into the above seeds In the culture medium, the seed culture solution was obtained by culturing at a rotating speed of 220 rpm and a shaking table at 30° C. for 24 hours. The seed culture solution was connected to the fermentation medium at a 10% inoculum amount, and the fermentation culture of the mutant QZ01 was obtained by culturing at a rotating speed of 220 rpm and a shaking table at 30° C. for 5 days. Centrifuge the 5L fermentation culture obtained from the fermentation culture, collect the fermentation cells, wash the cells 3 times with water, remove the washing liquid, and freeze-dry the cells overnight; add 2L methanol to the lyophilized fermentation cells, and ultrasonically treat them for 30 minutes, The extract was obtained by ...
Embodiment 2
[0033] Prepare 1L seed medium and 10L fermentation medium according to the seed medium and fermentation culture formula, and divide them into 250ml three-sided flasks, 50ml each, sterilize at 115℃ for 30min, insert the spores of mutant QZ01 into the above seeds In the culture medium, the seed culture solution was obtained by culturing at a rotating speed of 220 rpm and a shaking table at 30° C. for 24 hours. The seed culture solution was inserted into the fermentation medium at a 10% inoculum amount, and the fermentation culture of the mutant QZ01 was obtained by culturing at a rotating speed of 220 rpm and a shaking table at 30° C. for 5 days. Centrifuge the 10L fermentation culture obtained from the fermentation culture, collect the fermentation cells, wash the cells 3 times with water, remove the washing liquid, and freeze-dry the cells overnight; add 3L methanol to the freeze-dried fermentation cells, and ultrasonically treat them for 30 minutes. The extract was obtained by...
Embodiment 3
[0035] In vitro determination of antifungal activity and hemolytic toxicity of pimamycin derivatives
[0036] The in vitro antifungal activity is determined by Candida albicans as the indicator bacteria, and the growth of Candida albicans at different antibiotic concentrations is determined to obtain the MIC of the corresponding antibiotic 50 And MIC 90 value. First, the overnight culture solution of Candida albicans was inoculated into LB medium at a ratio of 1 / 10000, and 200μl / well was divided into 96-well plates. The DMSO solutions of the pimamycin derivative and pimamycin of different concentrations (0-500 μg / ml) were prepared and added to the above-mentioned LB medium at a ratio of 2%. Place the 96-well plate in an incubator at 34°C and incubate for 12 hours. Use a microplate reader to determine the OD under different concentrations of antibiotics 660 . Draw a bacteriostasis curve against the concentration of antibiotics from the measured data to get the MIC 50 With MIC 90 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com