Recombinant (alpha)-L-iduronidase, methods for producing and purifying the same and methods for treating diseases caused by deficiencies thereof
An iduronidase, iduronidase technology used in molecular biology, enzymology, biochemistry and clinical medicine to solve the problems of lack of donors, infeasibility, high morbidity and mortality in MPSI
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
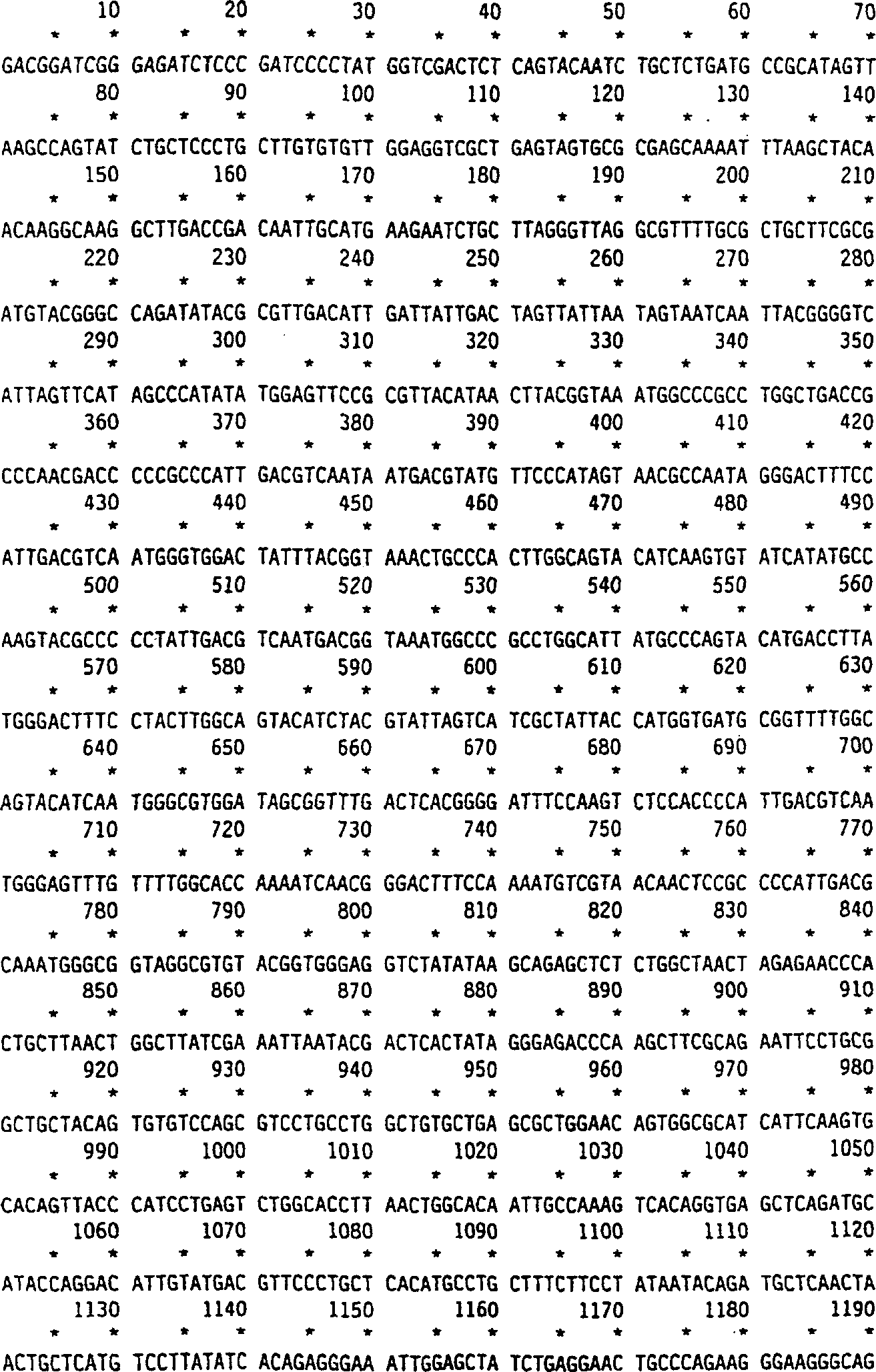
[0076] production of recombinant iduronidase
[0077] Standard techniques such as Sambrook et al. (1987) "Molecular Cloning: A Laboratory Manual"2 nd ed, Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. can be used to clone the cDNA encoding human α-L-iduronidase. The previously cloned human α-L-iduronidase cDNA was subcloned from bluescript KS as a HindIII-XbaI fragment into PRCCMV (InVitrogen). PCR amplification of bases 788-1372 (Tucker et al., Proc. Natl. Acad. Sci. USA, 78: 7684-7688 (1991)) of clone pRIR14.5 (Kakkis et al., Nucleic Acids Research, 16:7796 (1988)) An intron cassette derived from the Cot intron between exons 2 and 3 of murine immunoglobulin was augmented. The cassette included 136 bp of the 3' end of exon 2 and 5' of exon 3 The 242 bp of the end, which will remain in a correctly spliced cDNA. The ATG sequence is absent in the coding region of the intron box. The intron box is cloned into the 5' HindIII position of the α-L-iduronidase cDNA Point...
Embodiment 2
[0083] Recombinant α-L-iduronidase treatment is effective
[0084] Short-term intravenous administration of purified recombinant α-L-iduronidase to 9 MPSI dogs and 6 MPSI cats has shown that the enzyme is significantly An estimated 50% or more of them are absorbed. Although the liver and spleen absorbed the greatest amount of enzyme and had the best improvement in pathology, improvement in pathology and mucopolysaccharide content was observed in many but not all tissues. Specifically, cartilage, brain and heart valves did not improve significantly. Clinical improvement was observed in one dog over 13 months of long-term treatment, but other studies were limited to 6 months or less. All dogs and most cats that received the recombinant human enzyme developed antibodies to the human product. IgG antibodies are of the complement activating type (possibly canine IgG equivalents). This phenomenon was also observed in at least 13% of Gaucher disease patients treated with glucocer...
Embodiment 3
[0088] Recombinant α-L-iduronidase therapy effective in humans
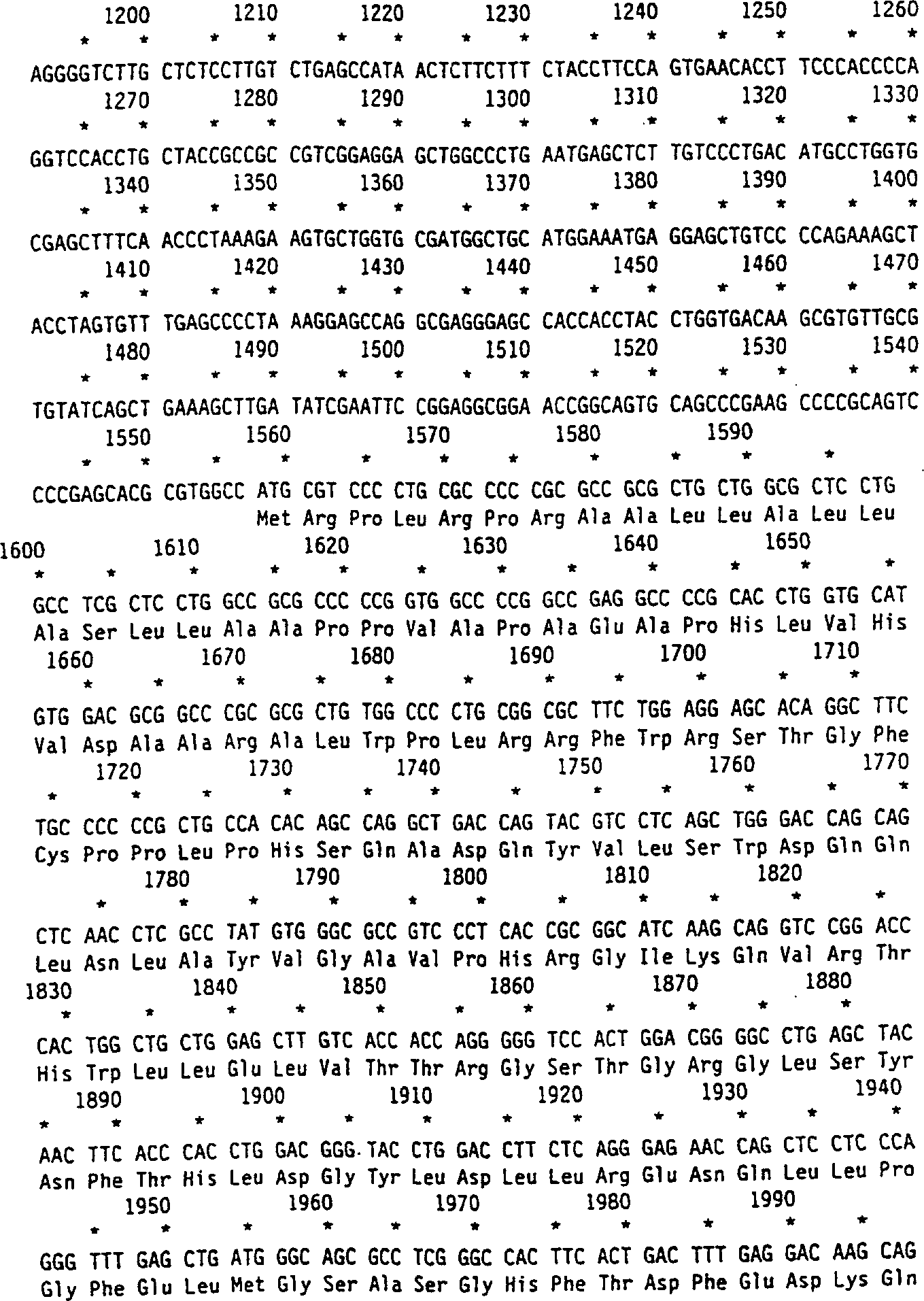
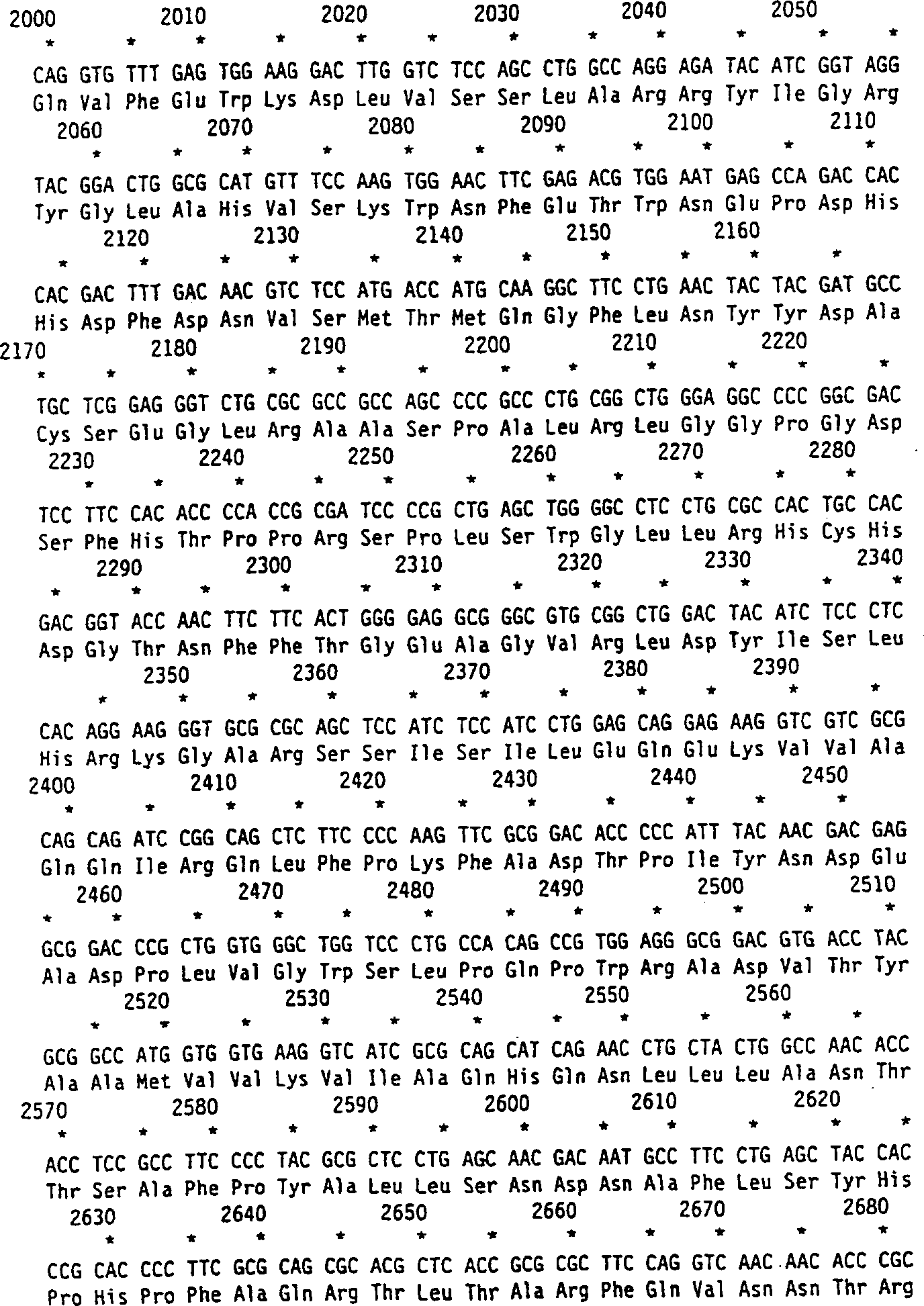
[0089] The human cDNA of α-L-iduronidase predicted a protein of 653 amino acids after cleavage of the signal peptide and a predicted molecular weight of 70,000 Daltons. Amino acid sequencing revealed the N-terminal alanine 26 giving a predicted protein of 629 amino acids. Human recombinant α-L-iduronidase has a histidine at position 8 of the mature protein. The predicted protein sequence contains 6 possible N-linked oligosaccharide modification sites. All of these sites are modified in the recombinant protein. The third and sixth positions have been shown to contain one or more mannose 6-phosphate residues that cause high affinity uptake into cells.
[0090] This peptide corresponds to amino acids 26-45 of human recombinant α-L-iduronidase, with an N-terminal alanine and the following sequence:
[0091] ala-glu-ala-pro-his-leu-val-his-val-asp-ala-ala-arg-ala-leu-trp-pro-leu-arg-arg
[0092] The recombinant e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com