Duplexed parvovirus vectors
a parvovirus and vector technology, applied in the field of parvovirus-based gene delivery vectors, to achieve the effects of improving the efficiency of transduction, rapid onset, and increasing the level of transgene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
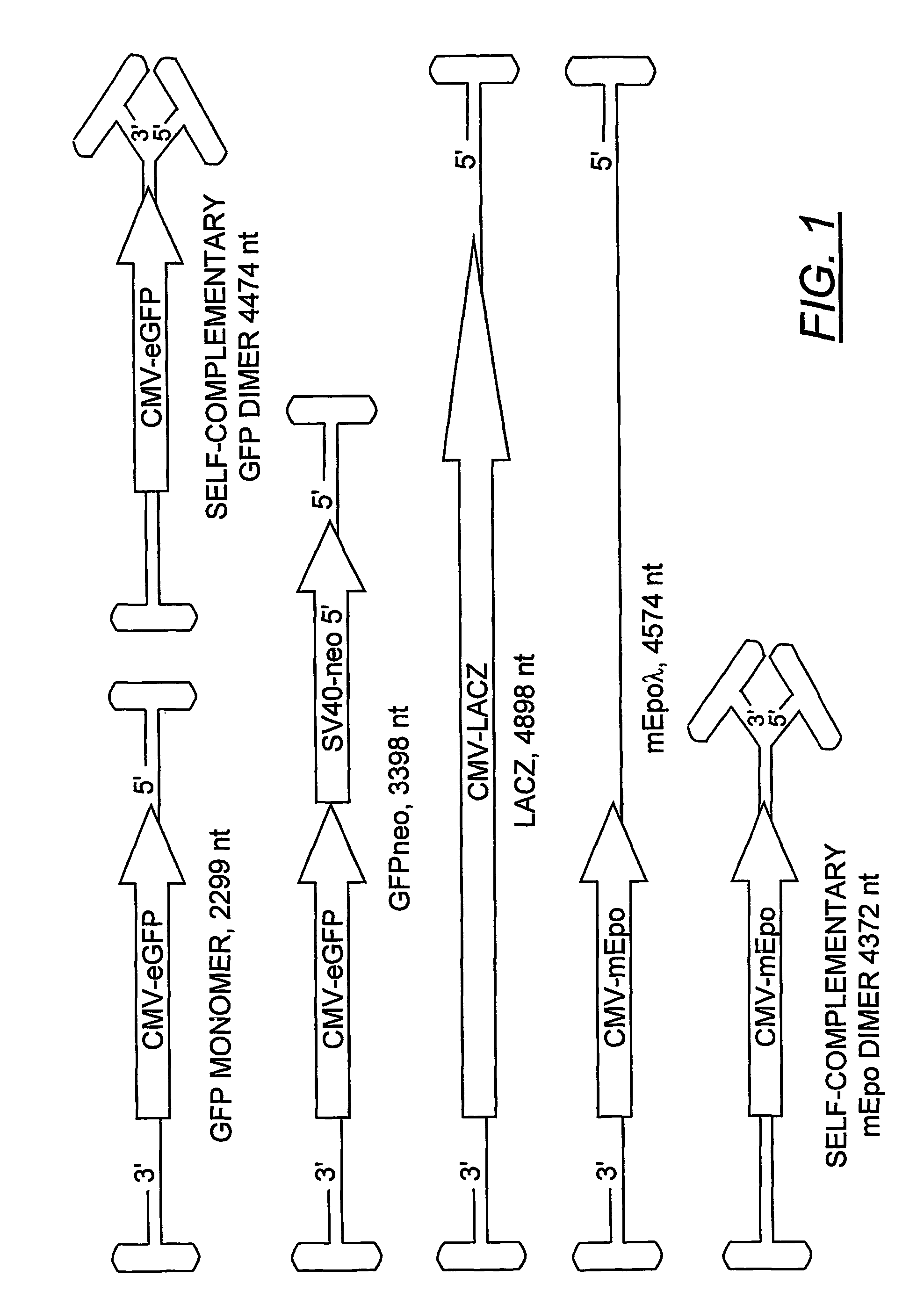
[0170]Plasmids. The rAAV plasmids expressing green fluorescent protein (GFP) were constructed from the previously described pTRBSUF-2 (a gift from Nick Muzyczka). First, the humanized GFP coding sequence was replaced with the enhanced GFP (eGFP) (Clonetech) to create the plasmid, pTR-CMV-GFPneo. This plasmid generated the rAAV-GFPneo vector. Second, the Sal I fragment containing the neo coding region and SV40 promoter was deleted to create pTR-CMV-GFP. The vector from this plasmid was referred to as rAAV-GFP in this report.
[0171]The plasmid, p43mEpo, a gift from Barry Byrne, contained the mouse erythropoietin gene under the control of the CMV promoter and generated a rAAV replicon (rAAVmEpo) of less than half the wtAAV length. A longer version of this construct (pmEpo-λ) was made by inserting the 2.3 kb Hind III fragment from λ phage into a Cla I site between the polyadenylation signal and the downstream AAV terminal repeat. The rAAV-LacZ vector was generated fr...
example 2
Generation of Duplexed Vectors
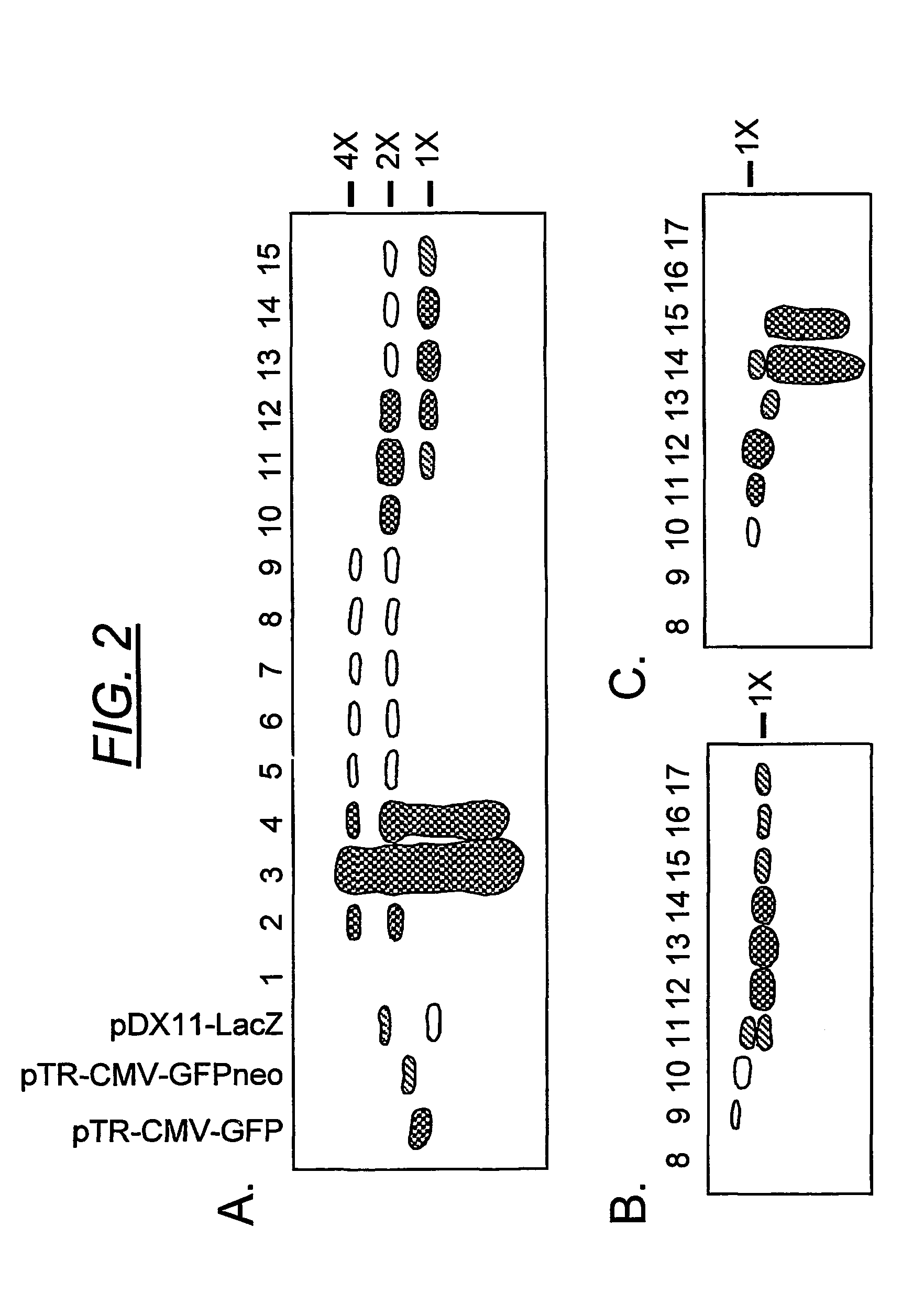
[0176]A rAAV plasmid construct (pTR-CMV-GFP), with a replicon size of 2299 nucleotides, was used to generate a viral vector stock (rAAV-GFP) by conventional methods. The predicted size of the dimeric replicative form of this vector was 4474 nucleotides (FIG. 1), which was 95.6% of the wt AAV genome length. The viral vectors were fractionated by isopycnic gradient centrifugation in CsCl and the vDNA content of each fraction was analyzed on alkaline agarose gels (FIG. 2). Phospholmager scans were used to quantify the vDNA specific bands from each fraction. Under denaturing conditions, the self-complementary dimer DNA (FIG. 2, panel a, fractions 10-13) ran at approximately twice the length of the monomeric genome. The hybridizing material in fractions 2-4 is unpackaged replicative form DNA that sediments at the bottom of the gradient. Although a DNase step was included in the vector purification (see methods), the treatment was not intended to be exhaustiv...
example 3
Transduction with Duplexed versus Monomeric Vectors and Effects of Ad co-Infection
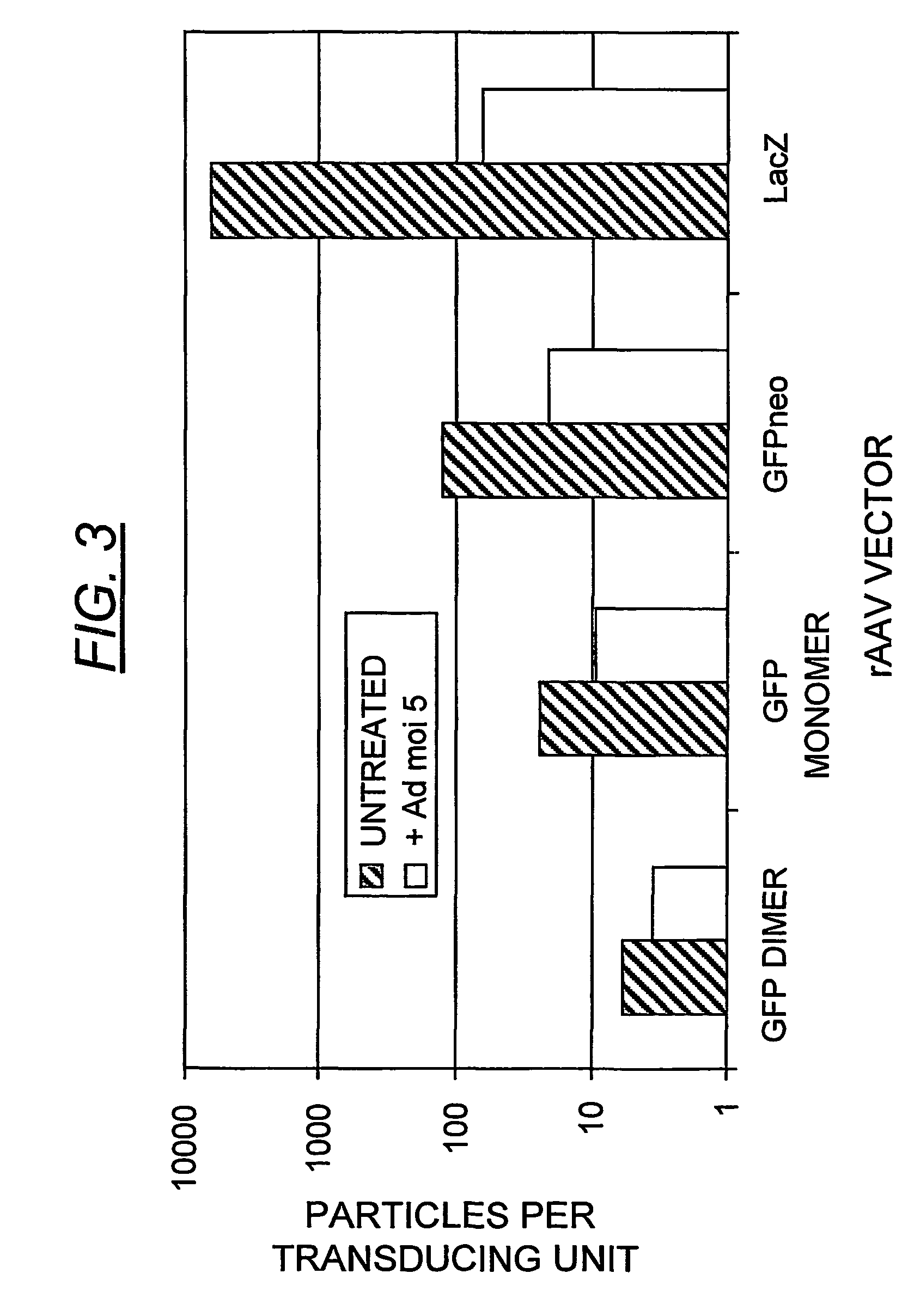
[0178]The transducing efficiency of the scAAV-GFP (FIG. 2, panel a, fraction 11) was compared with the homologous monomer (fraction 13), as well as the GFPneo and LacZ vectors (FIG. 2, panels b and c, fractions 13 and 12, respectively) in HeLa cells infected at low multiplicity (FIG. 3). The particle numbers were calculated from the specific, full-length vDNA Phospholmager signals in each fraction on the Southern blot, after correction for monomeric versus dimeric DNA copy number. Thus, each duplexed virus contains two copies of the transgene as a single molecule, in the inverted repeat orientation, while each monomeric particle contains one single-stranded copy.
[0179]The scAAV-GFP vector (fraction 11), containing approximately 90% dimer virus, yielded a 5.9:1 ratio of physical particles to transducing units, thus bearing out the prediction of high transducing efficiency. Fraction 13 from the same grad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com