Slow intraventricular delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0055]ASMKO mice are an accepted model of types A and B Niemann-Pick disease (Horinouchi et al. (1995) Nat. Genetics, 10:288-293; Jin et al. (2002) J. Clin. Invest., 109:1183-1191; and Otterbach (1995) Cell, 81:1053-1061). Niemann-Pick disease (NPD) is classified as a lysosomal storage disease and is an inherited neurometabolic disorder characterized by a genetic deficiency in acid sphingomyelinase (ASM; sphingomyelin cholinephosphohydrolase, EC 3.1.3.12). The lack of functional ASM protein results in the accumulation of sphingomyelin substrate within the lysosomes of neurons and glia throughout the brain. This leads to the formation of large numbers of distended lysosomes in the perikaryon, which are a hallmark feature and the primary cellular phenotype of type A NPD. The presence of distended lysosomes correlates with the loss of normal cellular function and a progressive neurodegenerative course that leads to death of the affected individual in early childhood (The Me...
example 2
“Intraventricular Infusion of rhASM in the ASMKO Mouse II”
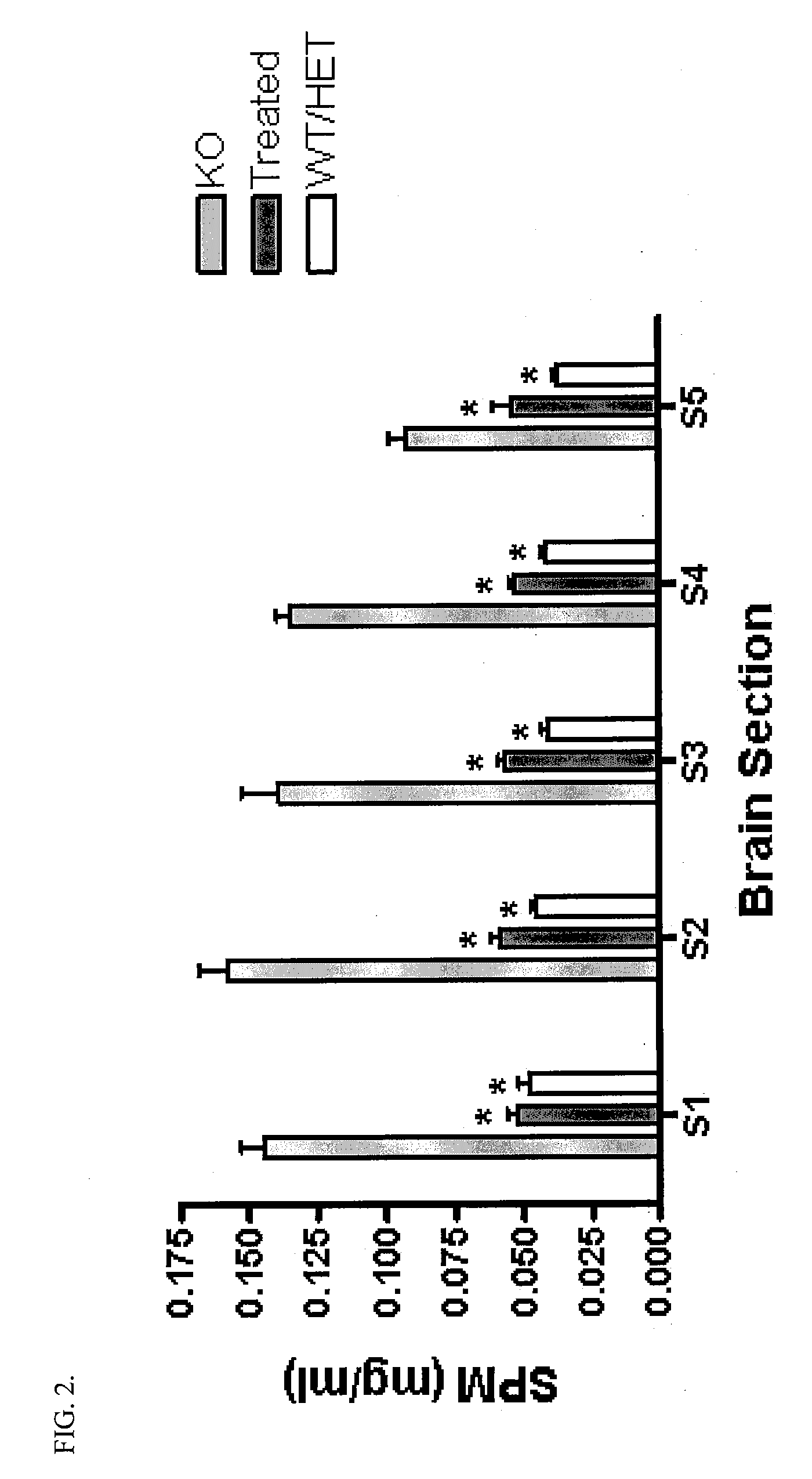
[0056]Goal: To determine what effect intraventricular infusion of rhASM has on storage pathology (i.e., sphingomyelin and cholesterol storage) in the ASMKO mouse brain
[0057]Methods: ASMKO mice were stereotaxically implanted with an indwelling guide cannula between 12 and 13 weeks of age. At 14 weeks of age mice were infused with 0.250 mg of hASM (n=5) over a 24 h period (˜0.01 mg / h) for four straight days (1 mg total was administered) using an infusion probe (fits inside the guide cannula) which is connected to a pump. Lyophilized hASM was dissolved in artificial cerebral spinal fluid (aCSF) prior to infusion. Mice were sacrificed 3 days post infusion. At sacrifice mice were overdosed with euthasol (>150 mg / kg) and then perfused with PBS or 4% parformaldehyde. Brain, liver, lung and spleen were removed and analyzed for sphingomyelin (SPM) levels. Brain tissue was divided into 5 sections before SPM analysis (S1=front of brain,...
example 3
“Intraventricular Delivery of hASM in ASMKO Mice III”
[0059]Goal: to determine lowest efficacious dose over a 6 h infusion period.
[0060]Methods: ASMKO mice were stereotaxically implanted with an indwelling guide cannula between 12 and 13 weeks of age. At 14 weeks of age mice were infused over a 6 period at one of the following doses of hASM: 10 mg / kg (0.250 mg; n=12), 3 mg / kg (0.075 mg; n=7), 1 mg / kg (0.025 mg; n=7), 0.3 mg / kg (0.0075 mg; n=7), or aCSF (artificial cerebral spinal fluid; n=7). Two mice from each dose level were perfused with 4% parformaldehyde immediately following the 6 h infusion to assess enzyme distribution in the brain (blood was also collected from these to determine serum hASM levels). The remaining mice from each group were sacrificed 1 week post infusion. Brain, liver, and lung tissue from these mice was analyzed for SPM levels as in study 05-0208.
TABLE 3GroupTreatmentnASMKO0.250 mg(10 mg / kg)12ASMKO0.075 mg(3 mg / kg)7ASMKO0.025 mg(1 mg / kg)7ASMKO0.0075 mg(.3 mg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com