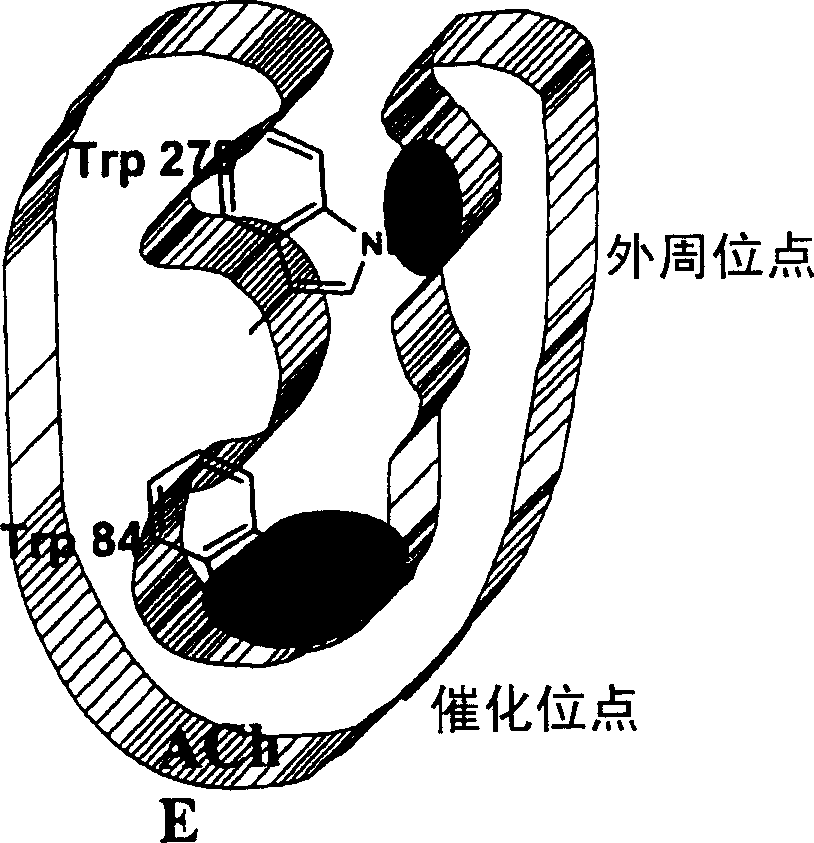
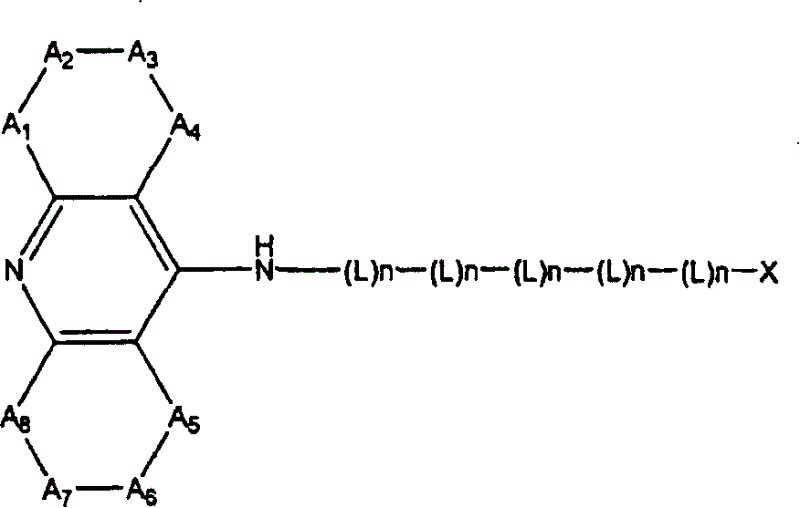
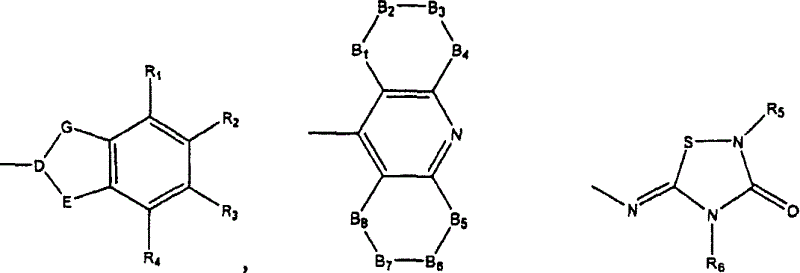
Dual binding site acetylcholinesterase inhibitors for the treatment of alzheimer's disease
A technology of ethyl and compound, applied in the field of a new family of compounds, which can solve the problems of increased neurotoxicity of βA fibrils, changes in enzyme biochemical and pharmacological properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
[0125] Example 1.5,6-dimethoxy-2-{[7-(1,2,3,4-tetrahydro-acridin-9-ylamino)heptylamino]-methyl}-2,3- Dihydro-inden-1-one
[0126]
[0127]To a stirred solution of 9-(7-aminoheptylamino)-1,2,3,4-tetrahydroacridine (134mg, 0.43mmol) in a mixture of ethanol:water 3:1 (3.5ml) at room temperature Paraformaldehyde (26 mg, 0.86 mmol) and 5,6-dimethoxy-2,3-dihydro-inden-1-one (83 mg, 0.43 mmol) were added. The pH was adjusted to 3 with 35% hydrochloric acid, and the mixture was refluxed for 24 hours. At the end of this period, the reaction mixture was cooled (25 °C), the solvent was removed under vacuum pressure, and the 2 CO 3 The residue was treated with saturated solution (3.5ml) and dichloromethane (5ml). The organic layer was washed with water (5ml) and dried (anhydrous Na 2 SO 4 ). The solvent was removed in vacuo and the residue was purified by preparative centrifugal thin layer chromatography. Elution with 5:1 ethyl acetate:methanol containing 1% aqueous ammonia pro...
Embodiment 25
[0131] Example 2.5,6-dimethoxy-2-{[6-(1,2,3,4-tetrahydro-acridin-9-ylamino)-hexylamino]-methyl}-2,3- Dihydro-inden-1-one
[0132]
[0133] According to the general method of Example 1, 9-(6-aminohexylamino)-1,2,3,4-tetrahydroacridine (96mg, 0.32mmol), paraformaldehyde (19mg, 0.64mmol), 5, 6-Dimethoxy-2,3-dihydro-inden-1-one (62 mg, 0.32 mmol) and 35% hydrochloric acid (pH=3) were refluxed for 24 hours. Purification by two preparative centrifugal thin layer chromatography using 10:1 ethyl acetate:methanol with 2% ammonia provided the title compound as a yellow slurry (8 mg, 5%).
[0134] 1 H-NMR (CDCl 3 , 300MHz, δ): 7.97(dd, 2H, J=8.1Hz), 7.56(ddd, 1H, J=8.1, 1.2Hz), 7.34(ddd, 1H, J=8.2, 1.2Hz), 7.13( s, 1H), 6.85(s, 1H), 3.94(s, 3H), 3.88(s, 3H), 3.55(t, 2H, J=7.0Hz), 3.30-3.19(m, 1H), 3.10-3.07 (m, 2H), 2.90-2.60(m, 7H), 1.89-1.68(m, 4H), 1.40-1.35(m, 2H), 1.28-1.11(m, 6H).
[0135] 13 C-NMR (CDCl 3 , 300MHz, δ): 207.1, 155.7, 151.2, 149.4, 149.3, 129.3, 128.7, 12...
Embodiment 3
[0137] Example 3.2-[6-(1,2,3,4-tetrahydro-acridin-9-ylamino)-hexylamino]-2,3-dihydro-indene-1,3-dione
[0138]
[0139] Add oxalic acid (1,2,3,4-tetrahydro-acridin-9-yl)-hexane-1,6-diamine (339mg, 0.87mmol) in ethanol:water 3:1 (3.5ml ) was added paraformaldehyde (26.4 mg, 0.88 mmol) and 2,3-dihydro-indene-1,3-dione (129 mg, 0.88 mmol). The pH was adjusted to 3 with 35% hydrochloric acid, and the mixture was refluxed for 24 hours. At the end of this period, the reaction mixture was cooled (25 °C), the solvent was removed under vacuum pressure, and the 2 CO 3 The residue was treated with saturated solution (3.5ml) and dichloromethane (5ml). The organic layer was washed with water (5ml) and dried (anhydrous Na 2 SO 4 ). The solvent was removed in vacuo and the residue was purified by preparative centrifugal thin layer chromatography. Elution with 10:1 to 3:1 ethyl acetate:methanol containing 1% aqueous ammonia provided the title compound as a yellow slurry (17 mg, 4.4%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com