Gene therapy for alzheimer's disease
A gene and disease technology, applied in the field of compositions for the treatment of Alzheimer's disease and other neurodegenerative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
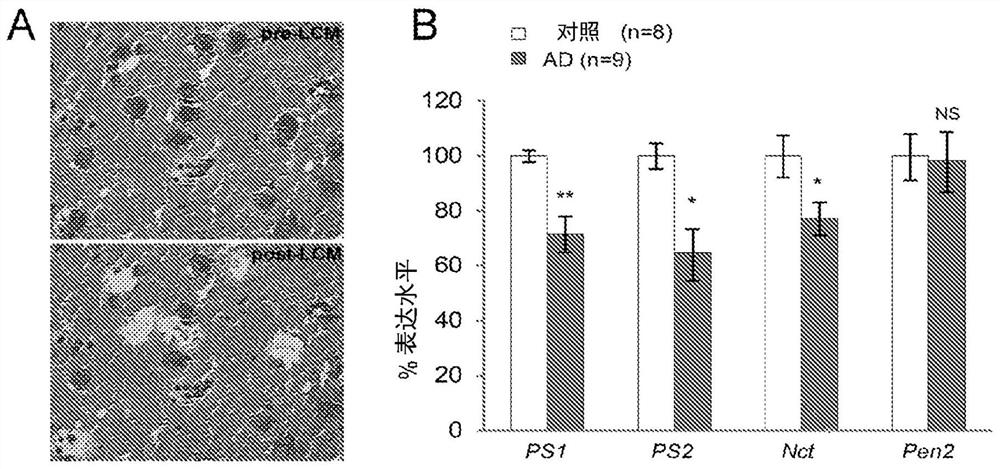
[0131] Example 1: Pyramidal neurons of patients with sporadic Alzheimer's disease (AD) exhibit reduced PSEN1 and PSEN2 mRNA levels
[0132] Presenilin (PS) protein is the catalytic subunit of γ-secretase, which is required for the production of Aβ peptides. Mutations in the presenilin-1 (PSEN1) and presenilin-2 (PSEN2) genes are associated with 90% of cases of familial Alzheimer's disease (FAD). PSEN mutations associated with FAD and temporofrontal dementia cause loss of presenilin expression and presenilin function, resulting in reduced γ-secretase activity (Xia et al., Neuron. 2015 Mar 4;85(5):967 -81; Watanabe et al., J Neurosci. 2012 Apr 11;32(15):5085-96; Brouwers et al., 2008 Ann Med 40(8):562–83).
[0133] Individual pyramidal neurons were collected using laser capture microdissection, and 300 neurons per human brain were pooled together for RNA preparation (PicoPure RNA Isolation Kit, Life Technologies KIT0204). qRT-PCR was performed using Platinum Taq DNA polymerase...
Embodiment 2
[0138] Example 2: Dose-Dependent Reduction of γ-Secretase Activity in MEFs Carrying Heterozygous and Homozygous PSEN1 Mutations
[0139] method
[0140] Generation of immortalized Psen mutant MEFs
[0141] Mouse embryonic fibroblasts (MEFs) carrying various Psen1 genotypes were maintained in medium supplemented with 10% FBS and 1% penicillin and streptomycin. In 6-well plates, 300,000 MEFs were immortalized by transfection with 1 μg of CMV-SV40. MEFs transfected with CMV-SV40 were compared to MEFs transfected with CMV-GFP (1 μg) that stopped dividing at about passage 7.
[0142] NdE and hPS1 transfection
[0143] To determine whether γ-secretase activity was impaired in various immortalized Psen mutant MEFs, CMV-NotchΔE (5 ng) was transiently transfected into 6-well plates using Lipofectamine LTX (ThermoFisher Scientific 15338030) following the manufacturer's instructions. MEF.
[0144] Approximately 24 hours after transfection, cells were lysed in RIPA buffer: 50 mM Tris...
Embodiment 3
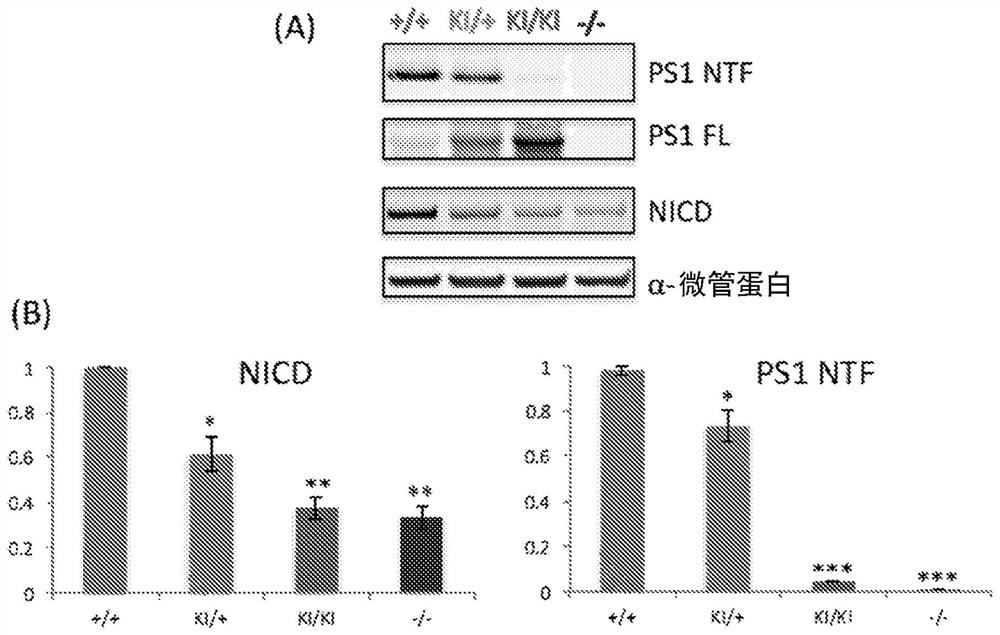
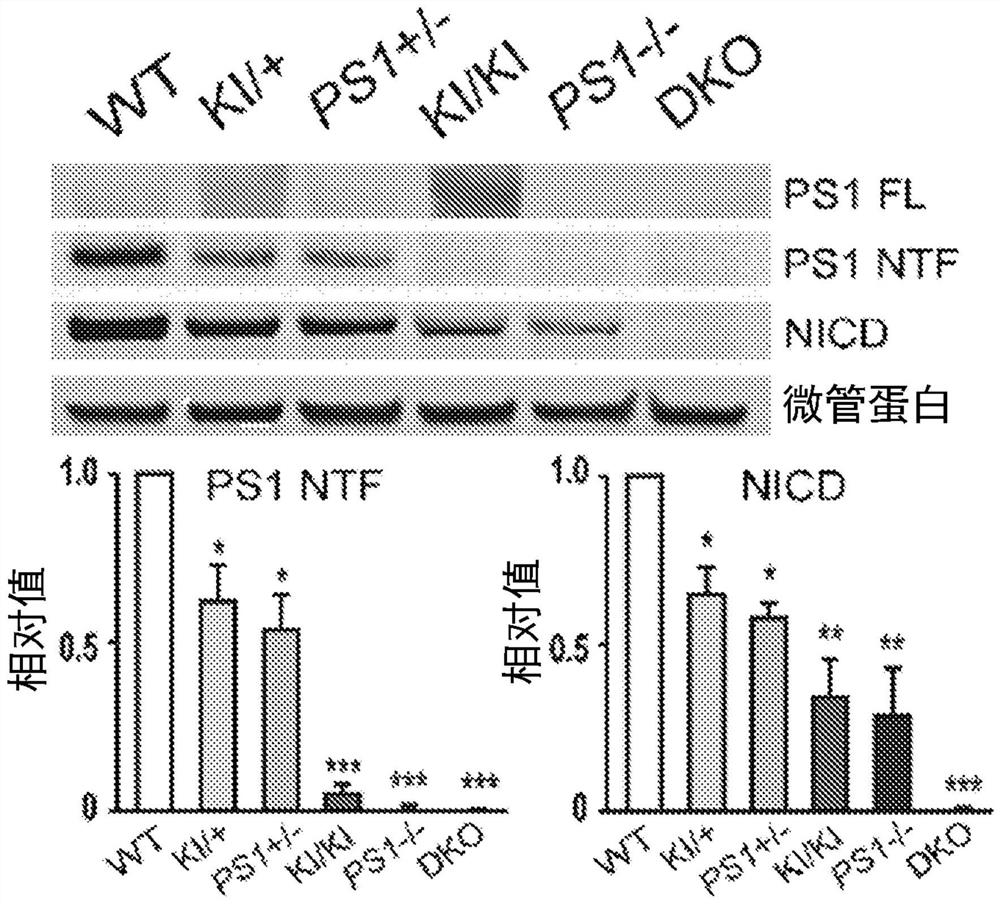
[0145] Example 3: Dose-dependent rescue of γ-secretase activity in MEFs with various PS genotypes: PS1 + / + , PS1 L435F / + , PS1 + / - , PS1 L435F / L435F , PS1 - / - and PS1 - / - ; PS2 - / -
[0146] To determine whether the reduced γ-secretase activity associated with PSEN1 mutations could be corrected by introduction of wild-type (WT) hPS, primary MEFs derived from embryos carrying various PS genotypes: PS1 + / + , PS1 L435F / + , PS1 + / - , PS1 L435F / L435F , PS1 - / - and PS1 - / - ; PS2 - / - (DKO). Immortalized MEFs were transiently transfected with CMV-NdelE, and γ-secretase activity was assessed by measuring the levels of NICD and PS1 NTF / CTF. NICD levels decreased in a PS1 dose-sensitive manner and were undetectable in DKO cells ( Figure 3A ). NICD level at PS1 L435F / L435F MEF (“L435F KI / KI” MEF) and PS1 - / - Decreased but detectable in MEF ( Figure 3A ), however by using the L435F KI / KI and PS1 - / - In vitro γ-secretase assays of embryonic brains did not detect de novo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com