Fujisu and its preparation method and application
A post-reaction technology of phexidine, applied in the field of medicine, can solve problems such as poor prognosis of surgery and unsatisfactory results of cholangiocarcinoma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
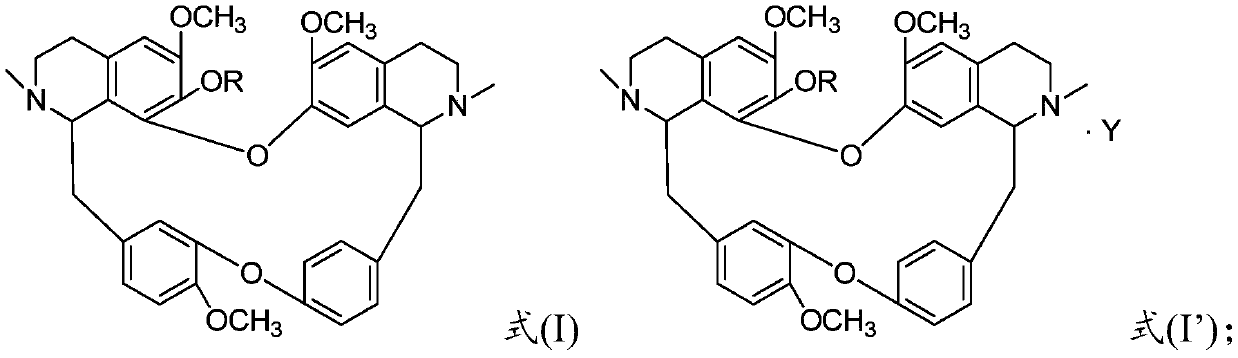
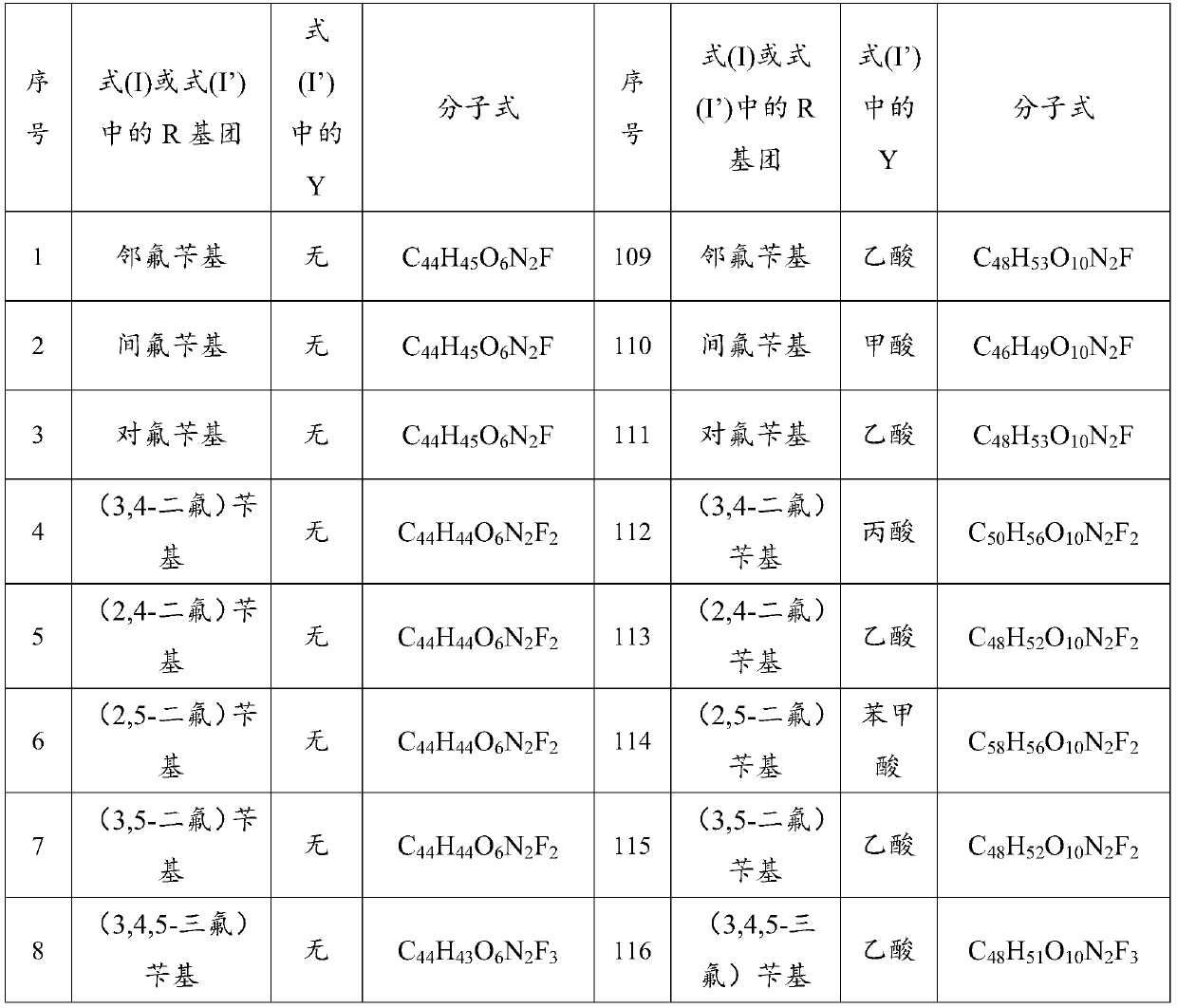
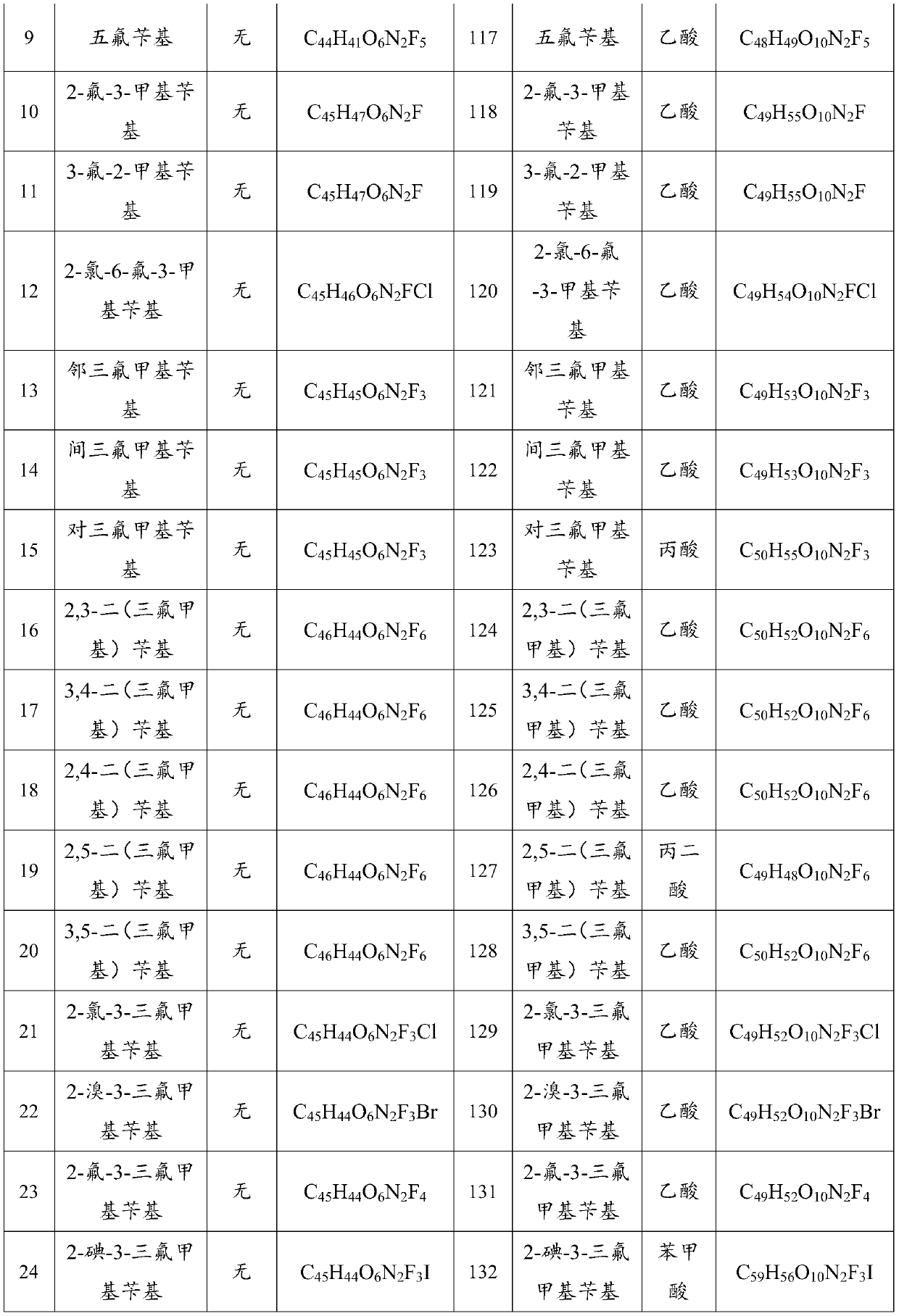
[0068] Weigh 18.30g of fangchinoline base and 0.72g of sodium hydride, dissolve it in 200mL of methanol and put it in a 500mL three-neck flask, then add 4.50g of o-fluorobenzyl chloride, stir and heat at 65°C, and keep stirring for 72h. TLC detects fangchinoline base For the whole reaction, distill methanol off, cool down to room temperature, add 80 mL of water, extract 3 times with dichloromethane (300 mL×3), trace the separation and purification process of the reaction and product by TLC, recover dichloromethane from the extract, and store the solid at 60°C After drying for 4 hours, 17.21 g of a light yellow powder was obtained. The melting point of the target product: 152.2-153.9°C, 13 C NMR (CDCl 3 ,100MHz)δ160.31,157.86,152.79,150.36,148.34,147.81,147.38,146.09,142.95,135.61,133.89,133.77,131.60,129.39,127.96,127.46,126.94,126.50,123.74,122.76,122.12,121.79,120.81,119.34 . TOF-HRMS: M / e (716.8477), molecular formula: C 44 h 45 o 6 N 2 F, namely compound 1 in Table ...
Embodiment 2
[0070] Weigh 18.30g of fangchinoline base, 2.10g of sodium ethoxide and dissolve it in 200mL of absolute ethanol in a 500mL three-neck flask, then add 5.80g of p-fluorobenzyl bromide, stir and heat at 78.5°C, keep stirring for 36h, and detect fangchinoline by TLC Lin alkali reacted completely, distilled out absolute ethanol, cooled to room temperature, added 80mL of water, extracted 3 times with chloroform (300mL×3), followed the separation and purification process of reaction and product by TLC, recovered chloroform from the extract, solid Dry at 60°C for 4 hours to obtain 17.85 g of a light yellow powder. The melting point of the target product: 153.1-154.7°C, 13 C NMR (CDCl 3 ,100MHz)δ163.55,161.11,153.75,151.39,149.43,148.78,148.52,147.13,143.95,136.68,135.12,134.68,133.31,132.65,130.15,130.09,130.01,128.43,128.26,128.10,123.12,122.82,121.91,120.23 . TOF-HRMS: M / e (716.8474), molecular formula: C 44 h 45 o 6 N 2 F, namely compound 3 in Table 1.
Embodiment 3
[0072] Weigh 6.10g of fangchinoline base and 1.40g of potassium carbonate, dissolve it in 100mL of propanol in a 500mL three-neck flask, then add 2.10g of 2,5-difluorobenzyl bromide, heat and stir at 98°C, and keep stirring for 24h, TLC Detect the entire reaction of fangchinoline base, evaporate the solvent under reduced pressure, cool down to room temperature, add 50 mL of water, extract 3 times with acetone (50 mL×3), trace the separation and purification process of the reaction and the product by TLC, and use anhydrous Na 2 SO 4 After drying for 8 hours, acetone was recovered, and the solid was dried at 60° C. for 4 hours to obtain 5.75 g of a light yellow powder. The melting point of the target product: 151.5-153.2°C, 13 C NMR (101MHz, CDCl 3 )δ153.76,151.22,151.09,149.42,148.73,148.63,148.46,147.08,143.80,136.34,135.32,134.87,134.73–134.56(m),132.65,130.13,128.75,128.60,123.91,123.29,122.80,121.89,120.17, 117.01, 116.83, 116.51, 116.23, 112.97, 111.61, 106.01, 72.85, 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com