Benzylisoquinoline non-depolarization muscle relaxant, and preparation method and use thereof
A technology of solvent and mivacurium chloride, which is applied in organic chemistry, pharmaceutical formulations, muscular system diseases, etc., can solve the problems of complex preparation process, poor stability, easy degradation, etc., and achieve simple preparation process, good stability, and reproducible good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0066] Weigh 250g 6,7-dimethoxy-2-(3-hydroxypropyl)-2-methyl-1-[(3,4,5-trimethoxyphenyl)methyl]-1,2 , 3,4-tetrahydroisoquinoline chloride, add 6L of 1,2-dichloroethane, and heat to 70°C to dissolve. Add 50 g of (E)-4-octenedoyl chloride, stir the reaction at room temperature for 19 hours, and concentrate under reduced pressure to remove the solvent. Add 2.5 L of chloroform to the obtained residue to dissolve, extract three times (3×3.5 L) with 5% sodium chloride solution, and concentrate the organic phase under reduced pressure to obtain solid mivacurium chloride.
Embodiment 1
[0069] Take 20 g of mivacuronium chloride obtained in Preparation Example 1, add 40 mL of methanol, and heat to 60° C. to dissolve under stirring to obtain a mivacuronium chloride solution. The obtained mivacurium chloride solution was slowly added into 600 mL of diethyl ether, and the stirring was continued at room temperature until no solid was precipitated, then the temperature was lowered to 15° C. and the stirring was continued for 2 hours. After filtration, the filter cake was dried under reduced pressure at 60° C. for 8 hours to obtain 18.7 g of white solid, with a yield of 93.5% and a purity of 95.0%.
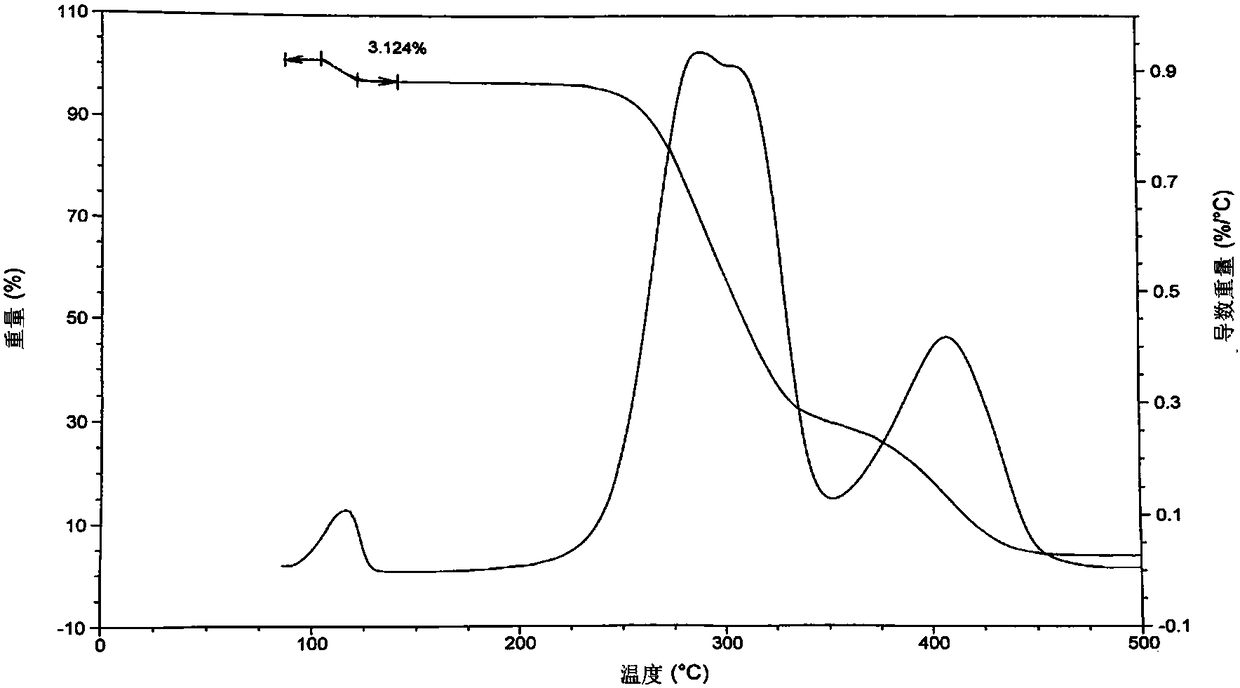
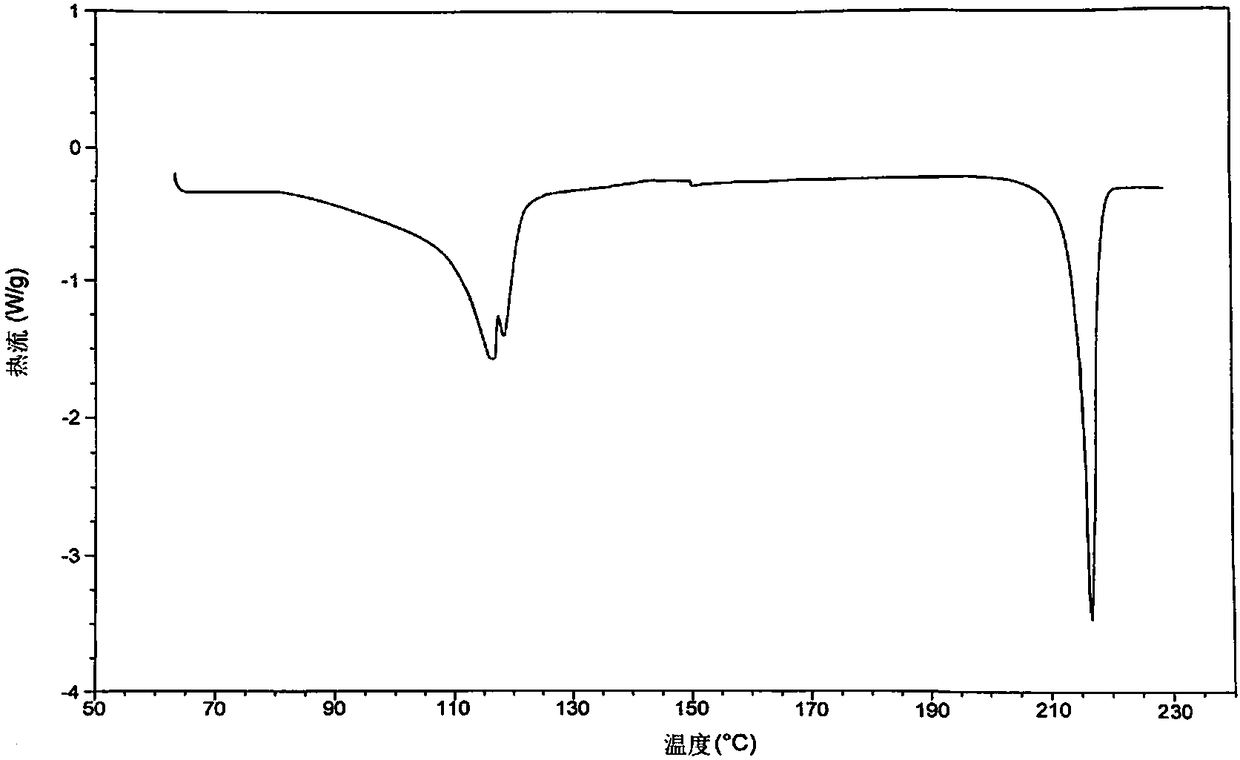
[0070] Structure Determination of Mivacurium Chloride Hydrate I:
[0071] (1) thermal analysis: get the white solid of embodiment 1 gained and carry out TGA (thermogravimetric analysis) and DSC (differential scanning calorimetry) test, gained result is respectively as follows figure 1 and figure 2 shown.
[0072] Thermal analysis results show that the white solid ...
Embodiment 2
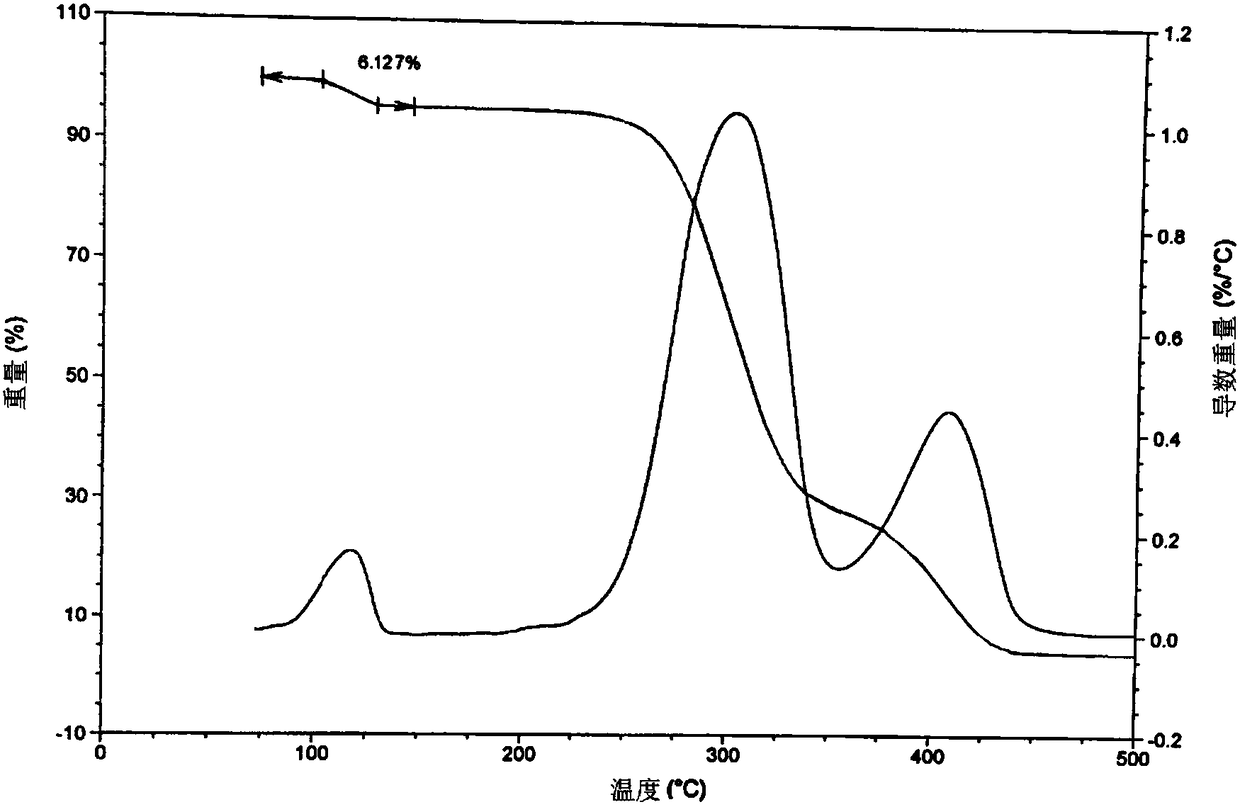
[0083] Take 20 g of mivacuronium chloride obtained in Preparation Example 1, add 30 mL of dichloromethane, and heat to 35° C. to dissolve under stirring to obtain a mivacuronium chloride solution. The obtained mivacurium chloride solution was slowly added into 400 mL of methyl tert-butyl ether, and stirring was continued at room temperature for 5 hours. After filtration, the filter cake was dried under reduced pressure at 30° C. for 8 hours to obtain 18.84 g of white solids with a yield of 94.2% and a purity of 95.23%. The water content of the product was 3.15% by weight.
[0084] The test results such as thermal analysis, elemental analysis, loss on drying, and moisture determination of the resulting product show that what the present invention obtains is Mivacurium Chloride Hydrate I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com