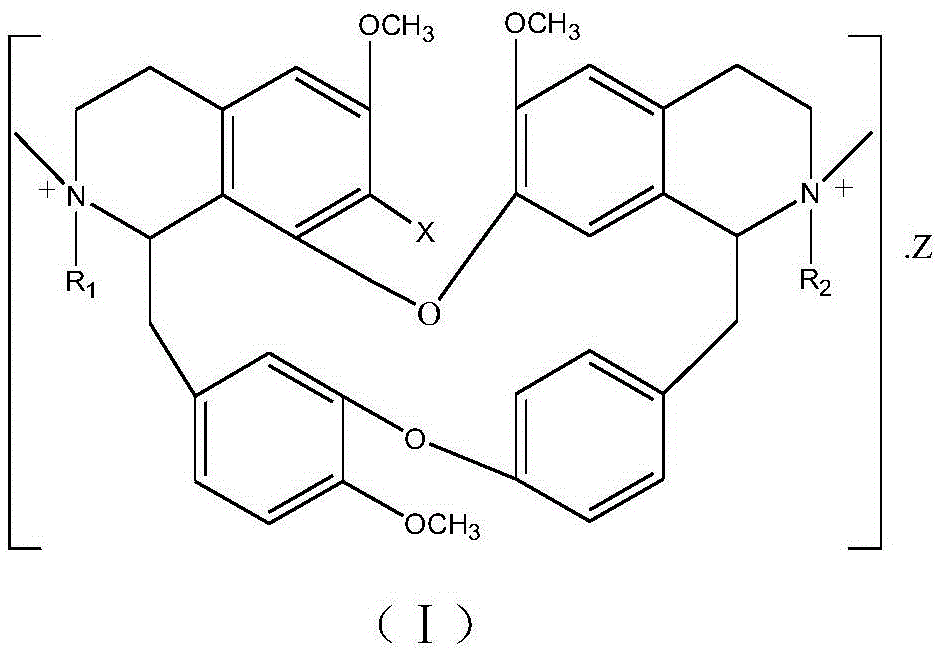
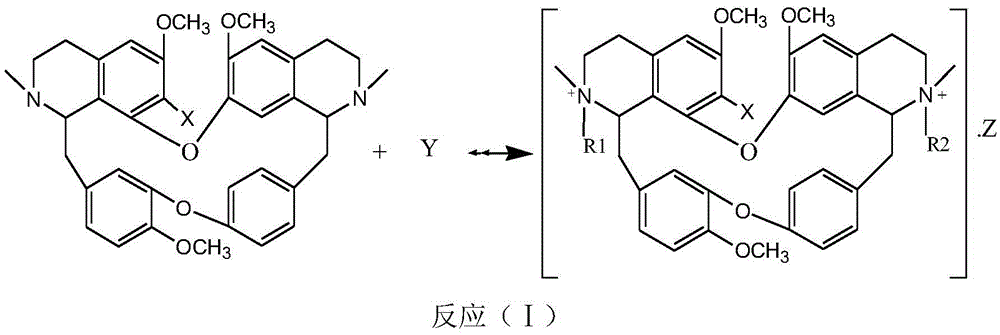
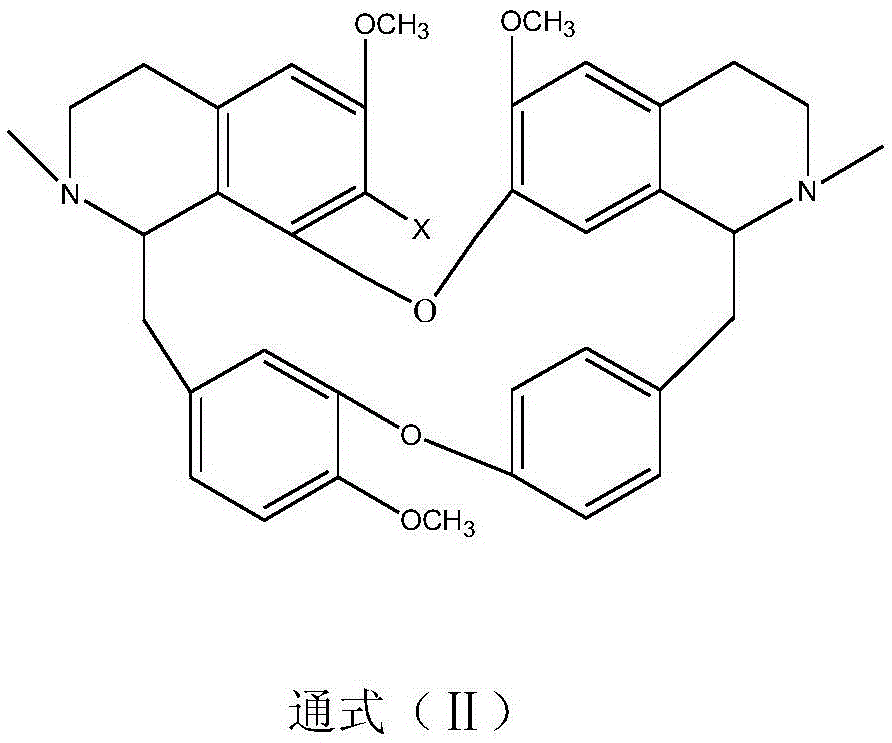
A kind of bisbenzylisoquinoline quaternary ammonium salt and its preparation method and application in the preparation of antitumor drugs
A technology of bisbenzylisoquinoline and quaternary ammonium salts, which is applied in the direction of antineoplastic drugs, drug combinations, and pharmaceutical formulations, and can solve problems such as lack of access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 6.10g of 7-hydroxybisbenzylisoquinoline (general formula ⅡX=OH), 3mL of methyl 2-chloroacetate and 1.50g of potassium carbonate, dissolve them in 200mL of ethanol, add them to a 500mL three-necked flask, heat and stir until boiling, And insulated and stirred for 24 hours, evaporated ethanol under reduced pressure, cooled to room temperature, added 50mL of water, neutralized to neutral with 10% hydrochloric acid, extracted 3 times with chloroform (50mL×3), traced the separation and purification process of reaction and product by TLC, extracted Anhydrous Na for liquid 2 SO 4 After drying for 8 hours, the chloroform was recovered, and the solid was dried at 60° C. for 4 hours to obtain 5.87 g of a light yellow powder. The melting point of the target product: 147-148°C, 13 CNMR (75MHz, DMSO-d 6 ): δ21.61(C-4), 23.12(C-4’), 35.63(C-15), 41.67(C-15’), 49.72(-COO * CH 3 ), 53.48 (NCH 3 ), 54.12 (N'CH 3 ), 56.16(C-3), 56.24(C-3'), 59.70(6-OCH 3 ), 59.74 (6'-OCH 3...
Embodiment 2
[0036] Weigh 6.10 g of 7-hydroxybisbenzylisoquinoline (general formula IIX=OH), 5 mL of methyl 2-chloropropionate and 1.50 g of sodium carbonate, dissolve them in 200 mL of propanol, add them to a 500 mL three-necked flask, heat and stir until Boil, keep warm and stir for 12 hours, evaporate the solvent under reduced pressure, cool down to room temperature, add 50mL of water, neutralize to neutral with 10% hydrochloric acid, and extract 3 times with acetone (60mL×3), trace the separation and purification process of reaction and product by TLC , the extract was anhydrous Na 2 SO 4 After drying for 8 hours, acetone was recovered, and the solid was dried at 60° C. for 4 hours to obtain 5.65 g of a light yellow powder. The melting point of the target product: 145-146°C, 13 CNMR experiment, time-of-flight mass spectrometry: M / e (348.1077), molecular formula C 41 h 48 o 8 N 2 Cl 2 , namely compound 7 in Table 1.
Embodiment 3
[0038] Weigh 6.10g of 7-hydroxybisbenzylisoquinoline (general formula ⅡX=OH), 10.00g of methyl 2-iodooctadecanoate, measure 200mL of ethyl acetate and 10mL of triethylamine, and add them to a 500mL three-necked flask. Stir, keep the reaction at -20°C for 72 hours, neutralize with dilute hydroiodic acid until neutral, separate the liquids, evaporate the ethyl acetate phase under reduced pressure until the liquid volume is reduced to 1 / 4, cool to 5°C to crystallize overnight, and filter , TLC traced the separation and purification process of the reaction and the product, and the solid was dried at 60° C. for 4 hours to obtain 1.52 g of a pale yellow powder. The melting point of the target product: 77-78°C, 13 CNMR experiment, time-of-flight mass spectrometry: M / e (453.2880), molecular formula C 56 h 78 o 8 N 2 I 2 , namely compound 16 in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com