Application of benzylisoquinoline alkaloid and derivative synthetic gene Ac6OMT thereof
A technology of benzylisoquinoline and alkaloids, applied in the fields of plant genetic improvement, application, genetic engineering, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
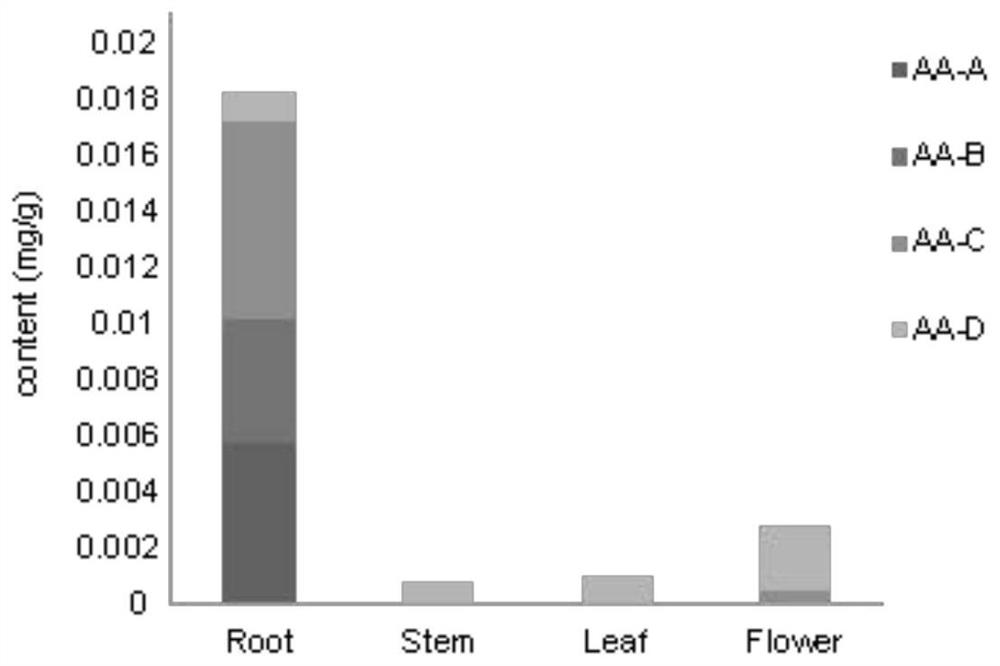
[0025] Determination of Aristolochic Acid Content in the Root, Stem, Leaf and Flower of Aristolochia militaris in Example 1
[0026] 1) Sample pretreatment
[0027] The four tissues of rhizome, leaf and flower were taken out from the -80°C refrigerator, and ground into fine powder using liquid nitrogen. Weigh 0.1 g of powder into a centrifuge tube, add 2 ml of 70% methanol for shaking, infiltrate at room temperature for 15 min, and then sonicate for 30 min. After centrifugation, the supernatant was collected, and the remaining precipitate was added to 2ml of 70% methanol for shaking, and ultrasonicated for 30min. The supernatant was collected after centrifugation and combined with the previously collected supernatant. After filtering through a 0.22μm filter membrane, wait for loading;
[0028] 2) UPLC detection of aristolochic acid A, B, C, D content
[0029] Use ACQUITYUPLC BEH C18 column (2.1X100nm, 1.7μm; Waters); detection wavelength: 254nm; column temperature: 30°C; f...
Embodiment 2
[0030] Example 2 Screening and Phylogenetic Analysis of Ac6OMT Gene Based on Aristolochia northernis Genome and Transcriptome Data
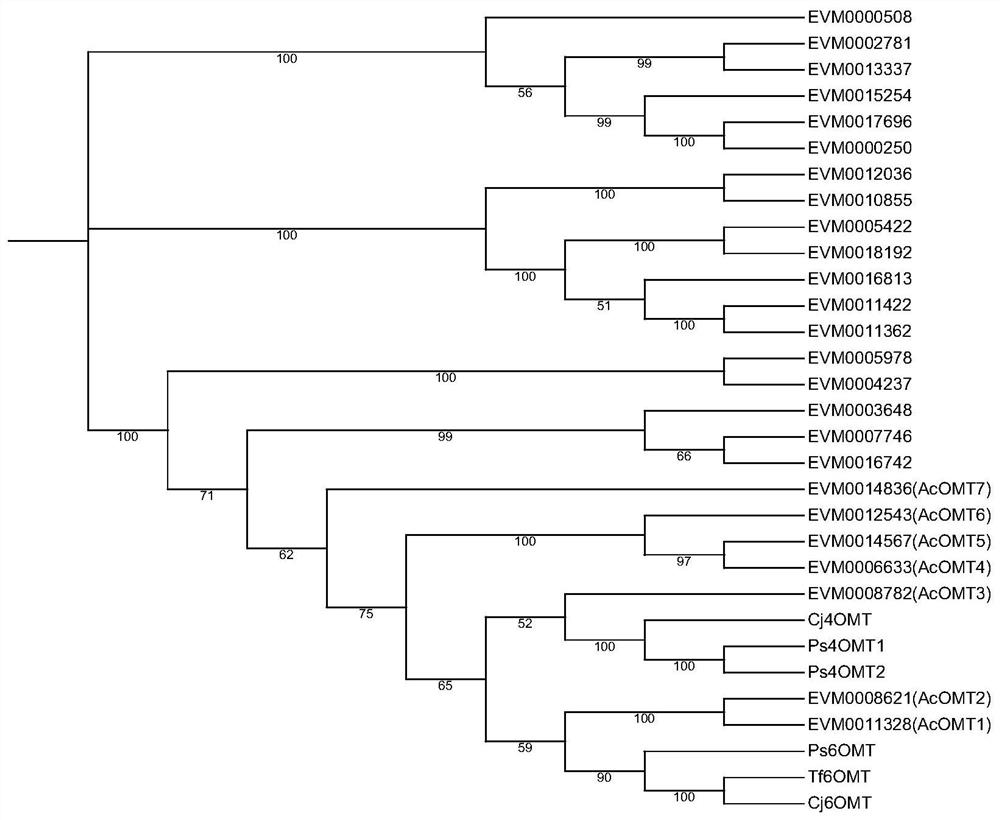
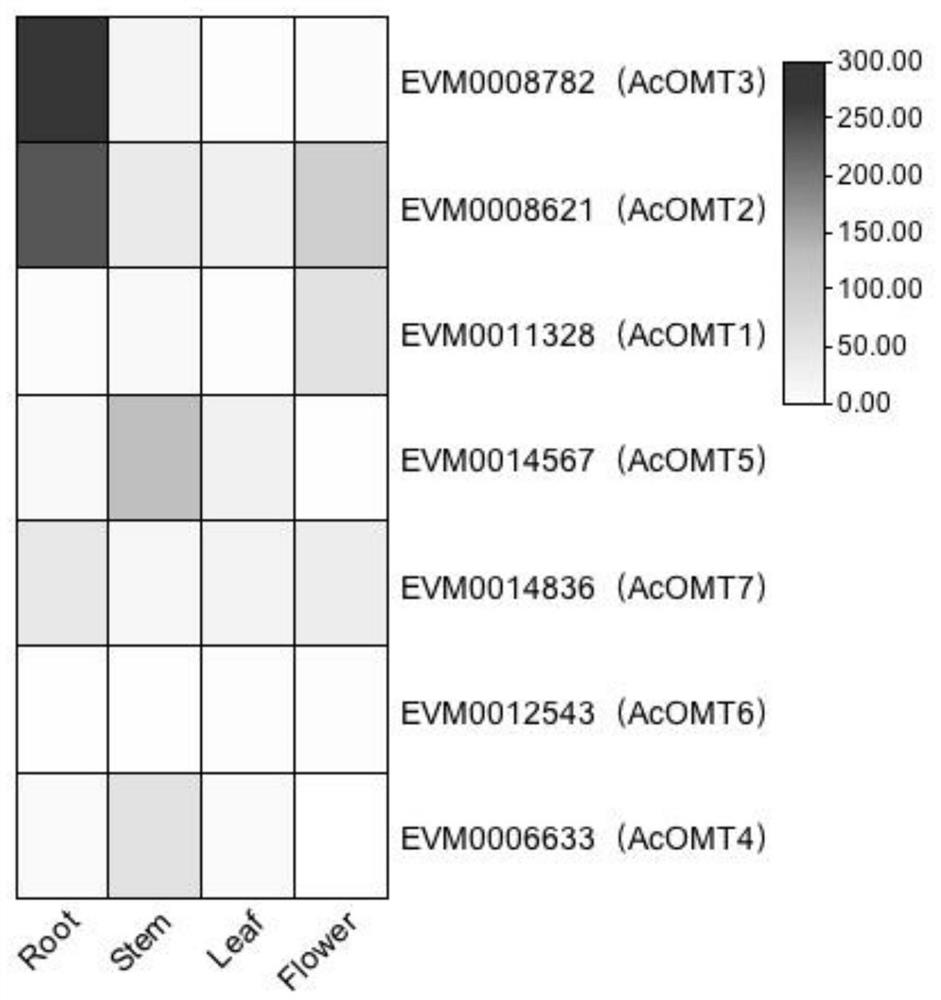
[0031] The OMT protein sequence of Arabidopsis thaliana was extracted from the Tair database, and Aristolochia miraculouse genome data were compared by BLASTP to identify AcOMTs of Aristolochia miraculouse. The 6 OMT protein sequences (Ps6OMT, Tf6OMT, Cj6OMT, Ps4'OMT1, Ps4'OMT2, Cj4'OMT) and AcOMTs of other species related to the synthesis of benzylisoquinoline alkaloids were searched in the Uniprot database and passed through MUSCLE comparison, construction of NJ phylogenetic tree, bootstrap selection repeated 1000 times. Such as figure 2 , focusing on 7 AcOMTs clustered together with 6 OMTs with known functions, AcOMT1-7. According to the transcriptome data, the expression FPKM values of these 7 AcOMTs in different tissues were extracted, and the heat map was drawn ( image 3 )Compare. It was found that in different tissues, the expressi...
Embodiment 3
[0032] Example 3 Cloning of Aristolochia miraculouse Ac6OMT gene
[0033] According to the open reading frame of the Ac6OMT gene sequence, primers were designed to amplify the full length of the gene, and the cDNA of Aristolochia militaris was used as a template to perform PCR amplification to obtain the nucleotide sequence of the Ac6OMT gene, such as SEQ ID No.1, and the full length of the gene was 1056bp. After translating the nucleotide sequence, the amino acid sequence of Ac6OMT was deduced, including 351 amino acid residues, such as SEQ ID No.2. Ac6OMT gene full-length amplification primers are shown in Table 1:
[0034] Table 1
[0035]
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com