Method for in vitro induction and amplification of human antigen nonspecific regulatory T cell
A non-specific and regulatory technology, applied in the field of biomedicine, can solve the problems of unstable phenotype and regulation, high cost of T cells, low induction efficiency, etc. type and regulation function to stabilize the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
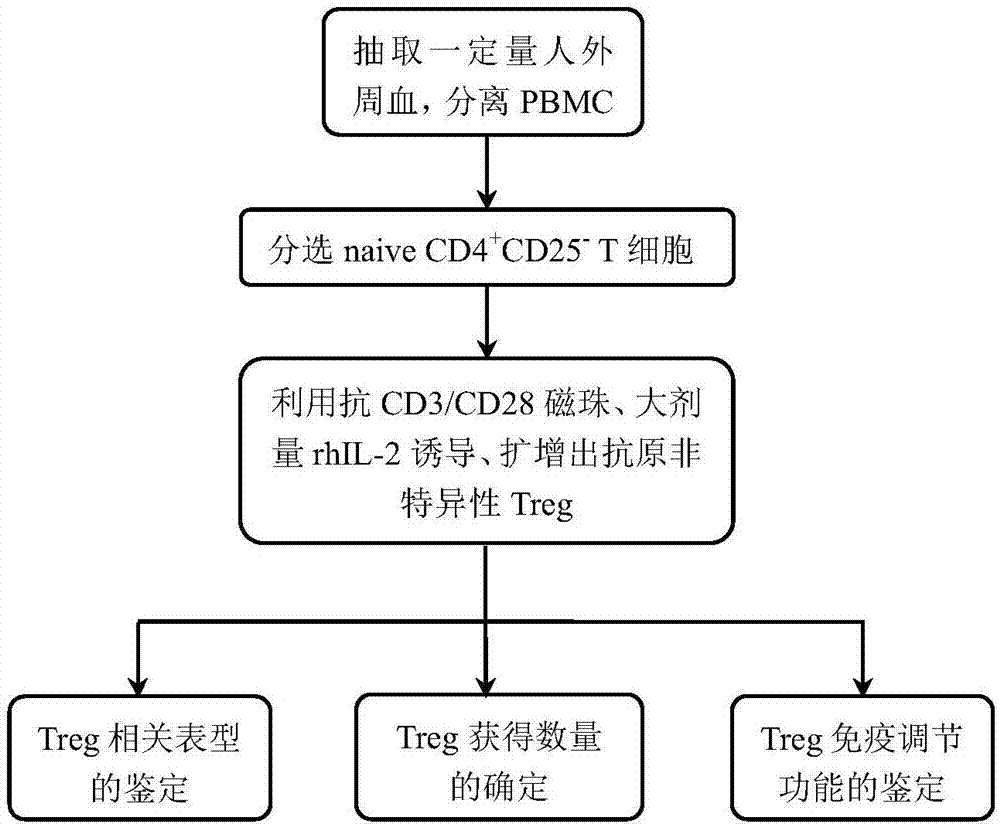
[0045] This example provides a method for inducing and expanding human antigen non-specific regulatory T cells in vitro, and the flow chart of the method is shown in the attached figure 1 As shown, the specific steps include:
[0046] 1: Recruit voluntary subjects, draw 20ml of peripheral blood, add an equal volume of normal saline to dilute and mix well, use human Ficoll lymphocyte separation medium, separate peripheral blood mononuclear cells (ie PBMC) by density gradient centrifugation, separate get 5.6×10 7 a PBMC;
[0047] Take 2.4×10 7 PBMC for For the sorting of T cells, the PBMCs were sequentially mixed with human naive T cells (i.e. T) Incubation with biotin-labeled antibody complexes from the sorting kit, avidin magnetic beads, and magnetic field purification to remove non-CD4 + T cells were incubated with magnetic beads coated with anti-CD25 antibody and purified by magnetic field to remove CD25 + T cells, resulting in CD4 + CD25 - CD45RA + T cells. Get ...
Embodiment 2
[0053] This example provides a method for inducing and expanding human antigen non-specific regulatory T cells in vitro, and the flow chart of the method is shown in the attached figure 1 Shown, concrete steps are basically the same as embodiment 1, difference is:
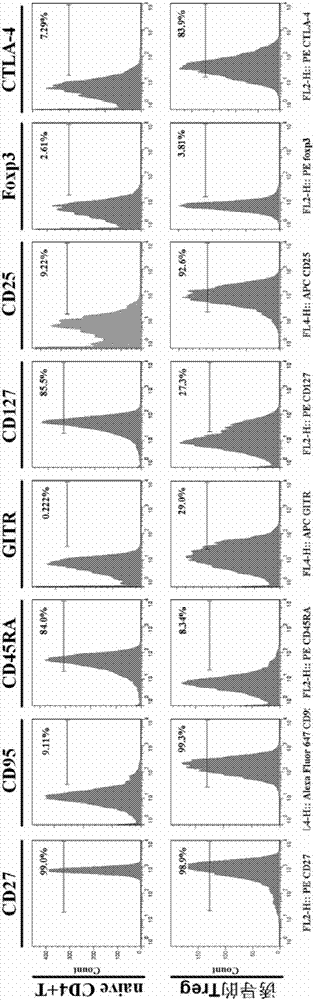
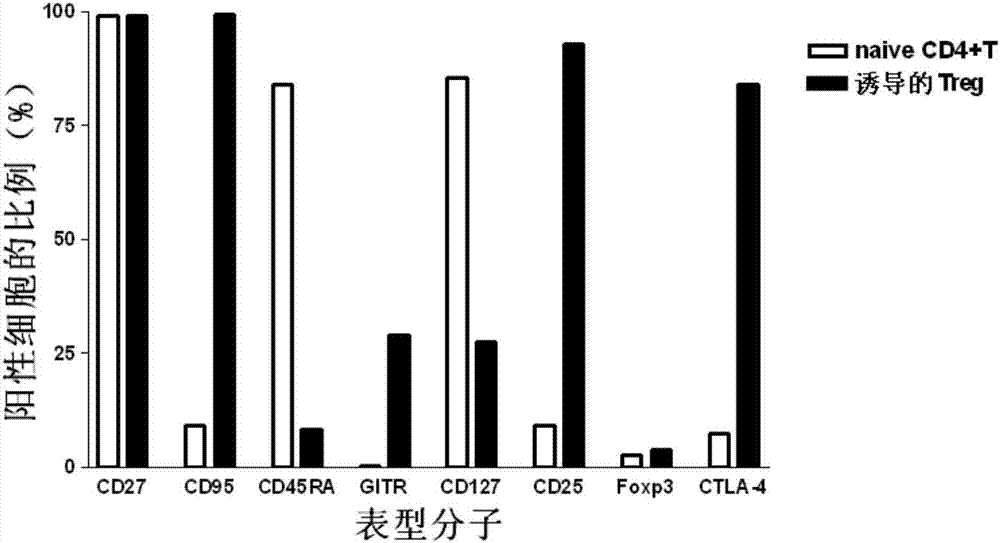
[0054] Step 2: Take 4.99×10 6 CD4 + CD25 - CD45RA +T cells were resuspended in 8ml of AMI-V CTS medium containing 10% autologous serum, added 3 times the amount of anti-CD3 / CD28 magnetic beads and rhIL-2 at a final concentration of 2000U / ml, and placed at 37°C with 5% In an incubator with a saturated humidity of carbon dioxide and 95% air, the culture was stimulated for 6 days, and rhIL-2 was supplemented every 3 days during the culture process, so that the final concentration of rhIL-2 was 2000 U / ml. The remaining cells were detected by flow cytometry for the expression of molecules such as CD27, CD95, CD45RA, GITR, CD127, CD25, Foxp3, and CTLA-4.
[0055] 3: After the induction, the magnetic field removes th...
Embodiment 3
[0058] This example provides a method for inducing and expanding human antigen non-specific regulatory T cells in vitro, and the flow chart of the method is shown in the attached figure 1 Shown, concrete steps are basically the same as embodiment 1, difference is:
[0059] Step 2: Take 2.9×10 6 CD4 + CD25 - CD45RA + T cells were resuspended in 4ml of AMI-VCTS medium containing 5% autologous serum, adding 1 times the amount of anti-CD3 / CD28 magnetic beads and rhIL-2 at a final concentration of 500U / ml, and placed at 37°C with 5% carbon dioxide and 95% air saturated humidity incubator, stimulated culture for 12 days, and added rhIL-2 every 3 days during the culture process, so that the final concentration of rhIL-2 was 500U / ml. The remaining cells were detected by flow cytometry for the expression of molecules such as CD27, CD95, CD45RA, GITR, CD127, CD25, Foxp3, and CTLA-4.
[0060] 3: After the induction, the magnetic field removes the anti-CD3 / CD28 magnetic beads, counts...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com