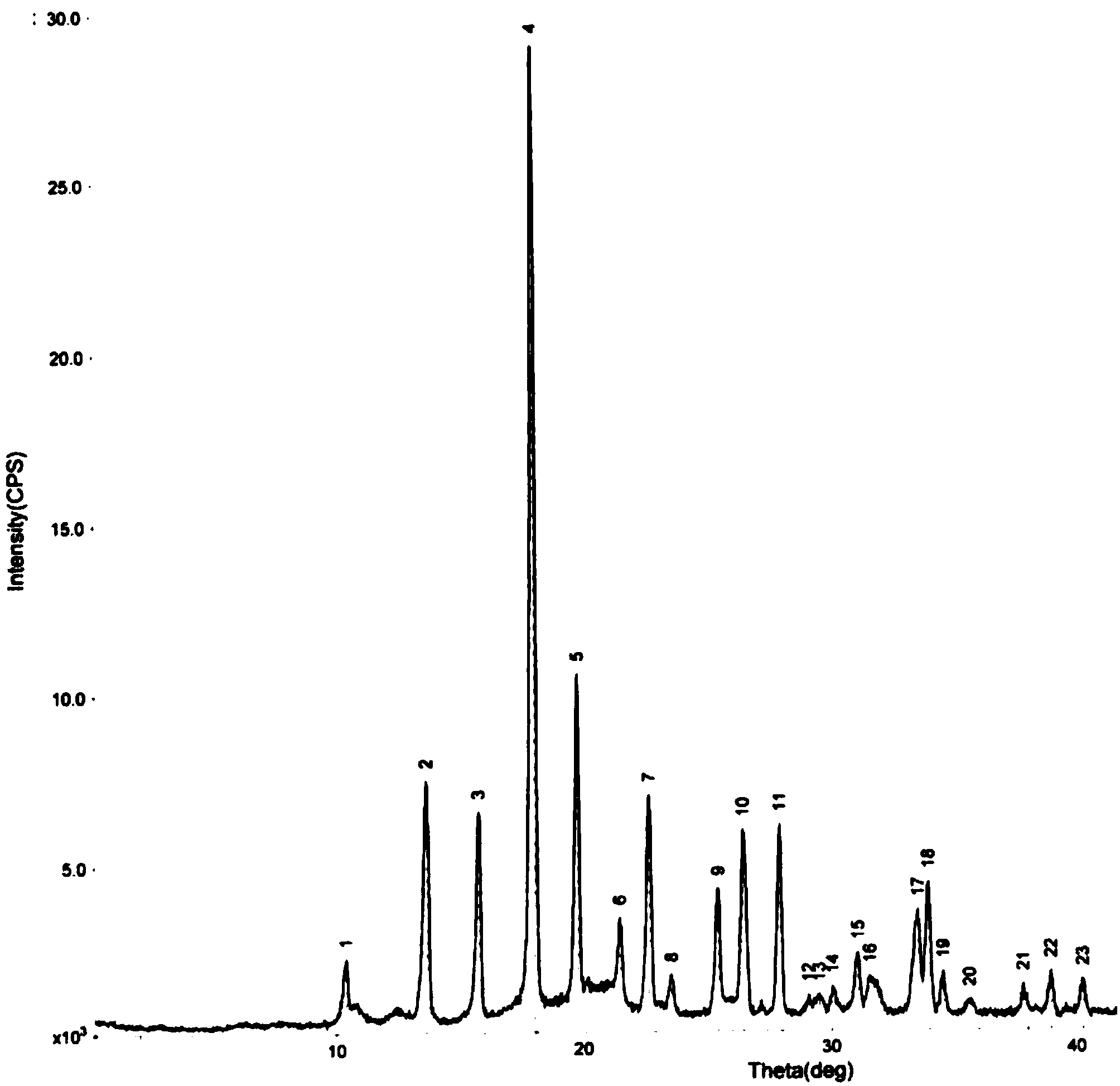
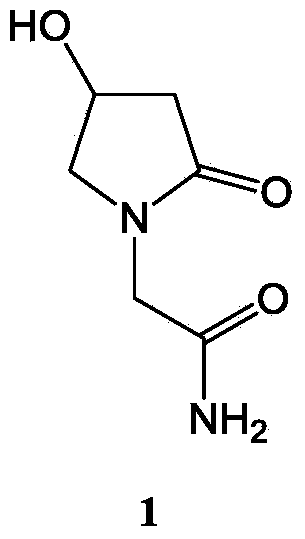
Oxiracetam freeze-drying preparation for injection and preparation method thereof
A technology for freeze-dried preparations and injections, which is applied in the field of pharmaceutical preparations and medicines. It can solve the problem of not revealing the existence of crystalline or non-crystalline forms of the final product state, no way of knowing stability, quality and clinical efficacy, and not involving lyophilized powder. Problems such as the form of solids exist, and the production process is green and environmentally friendly, the properties are stable, and the production cycle is shortened.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1 Preparation of oxiracetam freeze-dried preparation of the present invention
[0048]
[0049] Preparation method: Take 2400ml of water for injection, adjust the temperature to 60°C, add the prescribed amount of oxiracetam, sorbitol, and mannitol, stir for 30 minutes (rotation speed is 40Hz), cool down to 25°C, add 1.2g of medicinal charcoal, Stir and adsorb for 15 minutes, decarbonize and filter, adjust the pH value of the filtrate to 5.4 with 5% NaOH, add water for injection to 3000ml, fine filter with a 0.22μm microporous membrane, take the filtrate, fill it in a vial after passing the test, and transfer Freeze-dry in a freeze dryer, seal the rubber stopper, press the aluminum cover, and pack.
[0050] Wherein, the step of freeze-drying is:
[0051] (1) Pre-freezing: the temperature of the plate layer is dropped from room temperature to -15°C for 1 hour and kept for 6 hours; continue to drop to -30°C for 23 minutes and kept for 2.5 hours;
[0052] (2...
Embodiment 2
[0055] Embodiment 2 Preparation of oxiracetam freeze-dried preparation of the present invention
[0056]
[0057]
[0058] Preparation method: Take 2400ml of water for injection, adjust the temperature to 65°C, add the prescribed amount of oxiracetam, sorbitol, and mannitol, stir for 35 minutes (rotation speed: 40Hz), cool down to 30°C, add 2.4g of medicinal charcoal, Stir and adsorb for 20 minutes, decarbonize and filter, adjust the pH value of the filtrate to 5.0 with 5% NaOH, add water for injection to 3000ml, fine filter with a 0.22μm microporous membrane, take the filtrate, fill it in a vial after passing the test, and transfer To freeze-drying machine for freeze-drying (the freeze-drying steps are the same as in Example 1), sealing rubber stoppers, pressing aluminum caps, and packaging.
Embodiment 3
[0059] Embodiment 3 Preparation of oxiracetam freeze-dried preparation of the present invention
[0060]
[0061] Preparation method: Take 2400ml of water for injection, adjust it to 55°C, add the prescribed amount of oxiracetam, sorbitol, mannitol and sodium hydroxide, stir for 40 minutes (speed 40Hz), cool down to 27°C, add 0.6g medicinal Charcoal, stir and adsorb for 25 minutes, decarbonize and filter, adjust the pH value of the filtrate to 5.5 with 5% NaOH, add water for injection to 3000ml, fine filter with a 0.22μm microporous membrane, take the filtrate, and fill it in a vial after passing the test , moved to a lyophilizer for lyophilization (the lyophilization steps are the same as in Example 1), sealed with rubber stoppers, pressed aluminum caps, and packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com