Salts of sitafloxacin and pharmaceutical purposes thereof
A technology of sitafloxacin and hydrochloric acid, applied in the field of anti-infective drugs, can solve the problems of increasing production cost and difficulty, not having significant significance, and not solving the problems such as hygroscopic degradation of sitafloxacin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The synthesis of embodiment 1 sitafloxacin
[0064] According to Synthesis and stereochemical structure-activity relationships of chiral7-(7-amino-5-azaspiro[2.4]heptan-5-yl)-1-(2-fluorocyclopropyl)quinolone antibacterial agents (J Med Chem 1994, 37, 20, 3344 .) The disclosed method synthesizes sitafloxacin.
[0065] 1 HNMR (DMSO-d 6 )δ
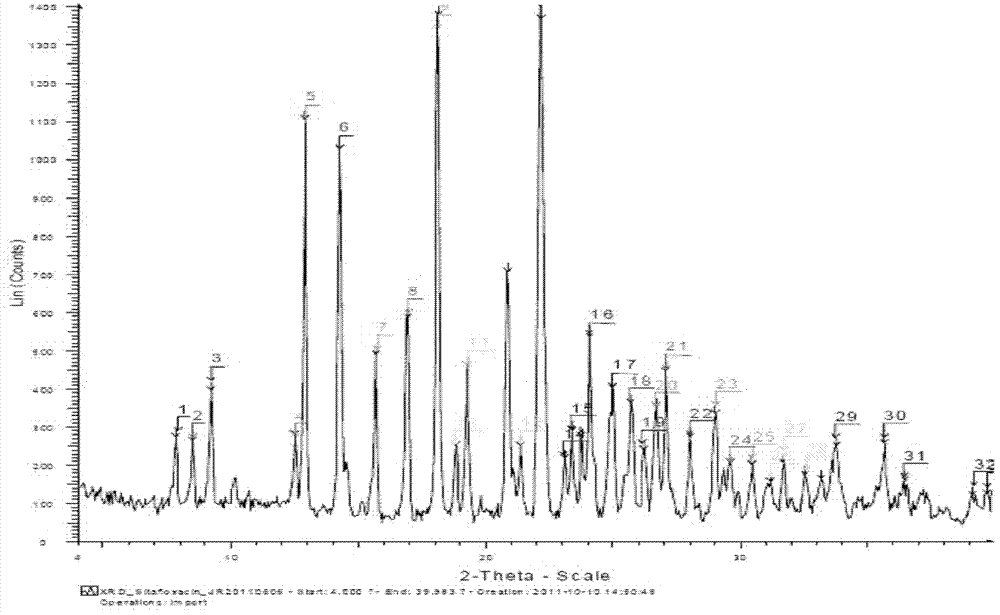
[0066] 0.43-0.46(m, 1H), 0.55-0.61(m, 2H), 0.79-0.83(m, 1H), 1.23(dm, 1H, J=27Hz), 3.06(t, 1H, J=5Hz), 3.25 -3.28(m, 1H), 3.35(d, 1H, J=7Hz), 3.83(d, 1H, J=7Hz), 3.92-3.96(m, 1H), 4.06-4.10(m, 1H), 4.50( dm, 1H, J = 64Hz), 7.74 (d, 1H, J = 14Hz), 8.47 (d, 1H, J = 2Hz) XRPD pattern shows crystals, and its X-ray powder diffraction pattern is at 2θ = 7.76°, 8.43° , 9.14°, 12.46°, 12.86°, 14.24°, 15.66°, 16.91°, 18.11°, 18.80°, 19.24°, 20.87°, 21.34°, 22.15°, 23.10°, 23.39°, 23.75°, 24.07°, 25.00 °, 25.70°, 26.22°, 26.71°, 27.07°, 28.04°, 29.04°, 29.62°, 30.48°, 31.22°, 31.73°, 32.57°, 33.22°, 33.82°°, 35.71°, 36.52°, 39.19° , the...
Embodiment 2
[0067] Preparation and characterization of embodiment 2 sitafloxacin hydrochloride
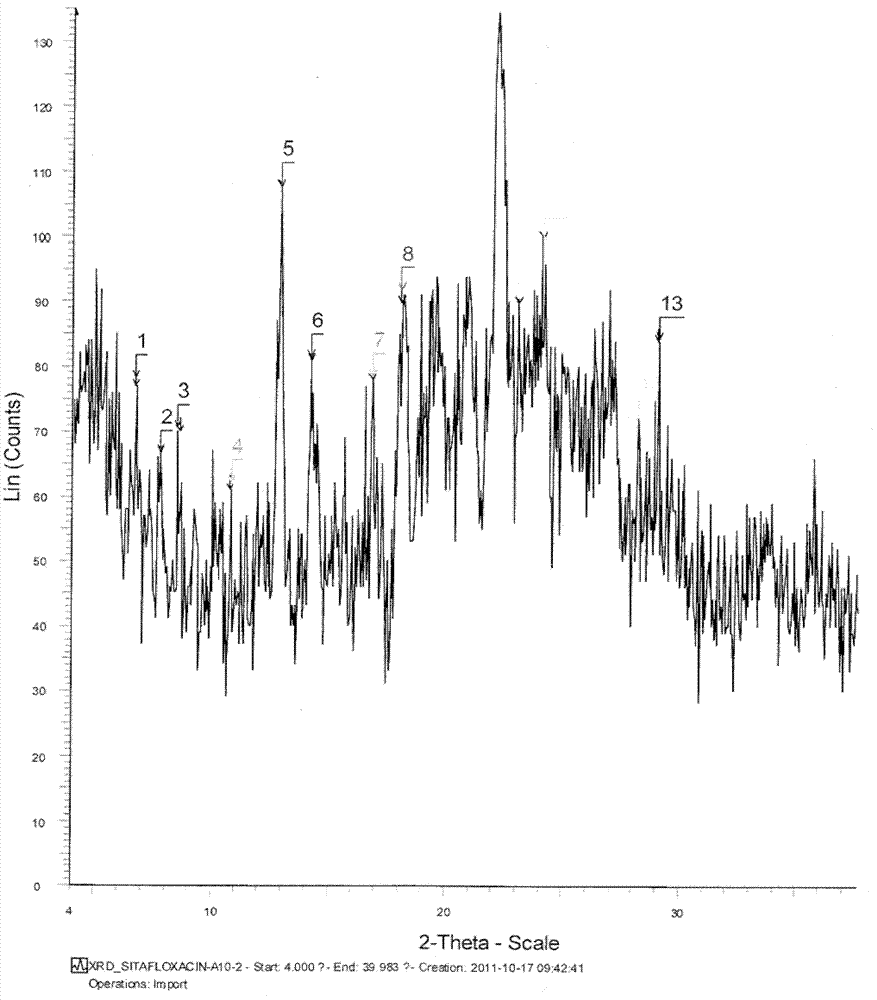
[0068] Weigh the obtained 100mg sitafloxacin (0.24mmol, 1eq), dissolve it in 15mL acetone, control the temperature at 30°C, stir until dissolved, add a hydrochloric acid solution containing 13.5mg hydrochloric acid (0.37mmol, 1.5eq) to the solution, stir reaction. After the reaction was finished, n-heptane was added to the reaction solution until the cloud point appeared. Reduce the temperature to room temperature at a rate of 5°C / min, filter, collect the solid, and dry under reduced pressure at 45°C overnight to obtain the obtained product. The obtained sitafloxacin hydrochloride was characterized by XRPD and DSC.
[0069] The melting point detected by DSC was 123.03°C.
Embodiment 3
[0070] Embodiment 3 Preparation and characterization of sitafloxacin tartrate crystal form I
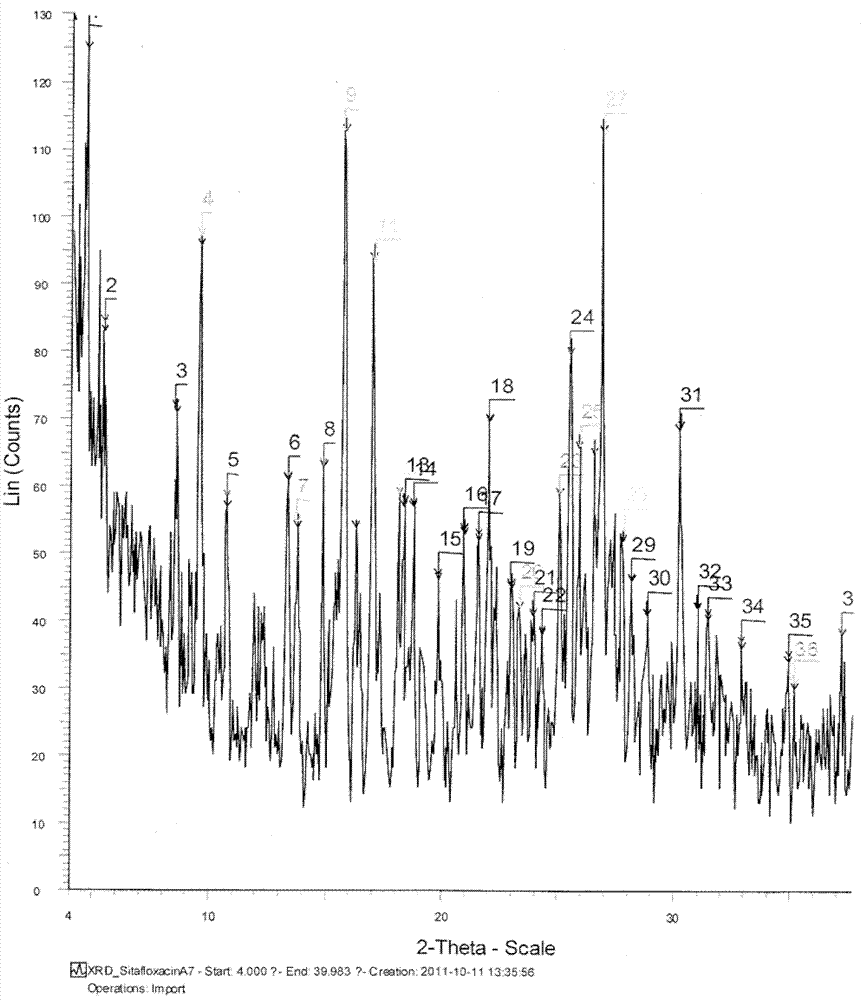
[0071] Weigh 100mg of sitafloxacin (0.24mmol, 1eq), dissolve it in 15mL of acetone, control the temperature at 35°C, stir until dissolved, add a solution containing 43.2mg of tartaric acid (0.29mmol, 1.2eq) to the solution, and precipitate . Reduce the temperature to room temperature at a rate of 5°C / min, filter, collect the solid, and dry under reduced pressure at 45°C overnight to obtain the obtained product. The obtained sitafloxacin hydrochloride was characterized by XRPD and DSC.
[0072] The melting point detected by DSC was 193.32°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com