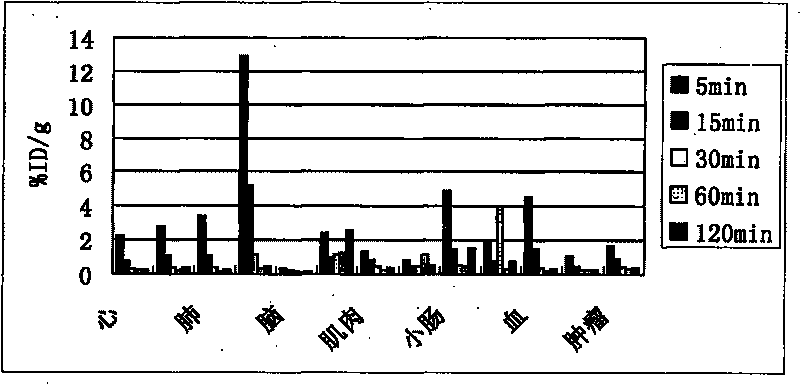
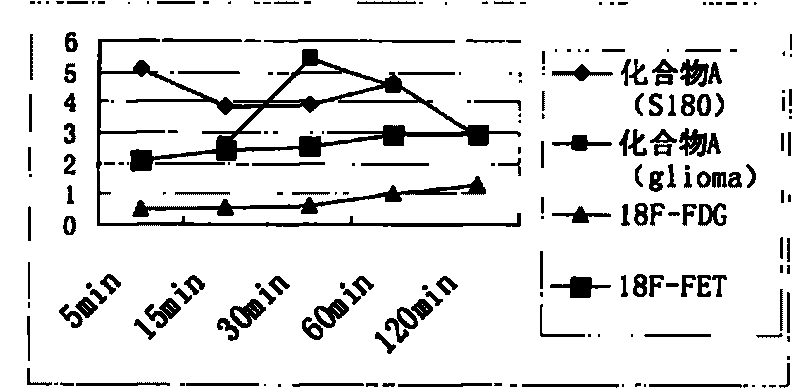
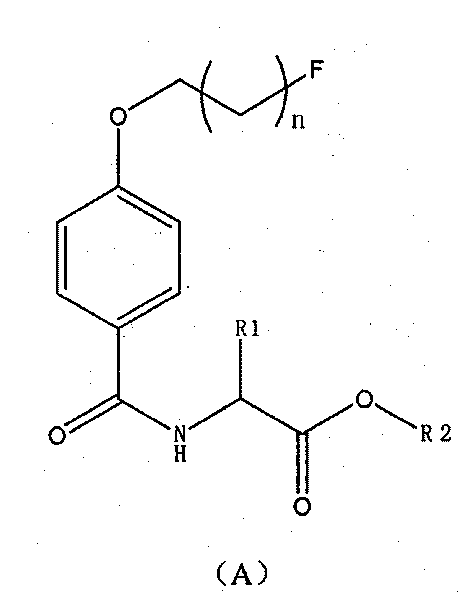
Novel 18F labeled amino acid derivatives, preparation method and application thereof in tumor imaging
A technology for amino acids and derivatives, which is applied in the field of new 18F-labeled amino acid derivatives, can solve the problems of complex preparation and purification, and achieve the effects of simple operation, good biological activity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1 formula C compound 4-(2-hydroxyethoxy) benzamide methyl acetate
[0069]
[0070] Amino acid methyl ester hydrochloride (3mmol) was dissolved in 20mL of dichloromethane, redistilled triethylamine (3mmol) was added in one go, stirred for 5min, and 4-(2-hydroxyethoxy)benzoic acid (2mmol) was added and HOBt (1-hydroxybenzotriazole) (2.2mmol), the reaction mixture was cooled to 0°C, and 5mL of DCC (2.2mmol) in dichloromethane was added dropwise. React overnight at room temperature, TLC shows that after the reaction is over, remove a large amount of white precipitate DCU by suction filtration, the organic phase is washed with water, saturated sodium bicarbonate aqueous solution, saturated sodium chloride aqueous solution, dried over anhydrous sodium sulfate, and the oil is obtained after spin-off solvent Object. Column chromatography, eluent (ethyl acetate: methanol 10: 1) to obtain a light yellow oil, add a small amount of ethyl acetate and petroleum ether m...
Embodiment 2
[0071] Example 2 Synthesis of 4-(2-p-toluenesulfonate ethoxy)benzamido amino acid methyl ester of compound of formula B.
[0072]
[0073] Dissolve the compound C (1 mmol) prepared above into 20 mL redistilled dichloromethane, add TEA (triethylamine) (1.5 mmol), cool in an ice bath to below 0°C, add DMAP (4-N, N-dichloromethane picoline) (0.2mmol) and p-toluenesulfonyl chloride (1.5mmol) of re-purification treatment, after continuing the low-temperature reaction for 30min, rising to room temperature and reacting overnight, TLC showed that the reaction was complete. After adding saturated aqueous sodium bicarbonate solution and fully stirring, the organic The layer was fully washed with saturated brine, dried over anhydrous sodium sulfate, and separated and purified by column chromatography.
Embodiment 3
[0074] Example 3 Synthesis of formula A compound 4-(2-fluoroethoxy)benzamido amino acid methyl ester.
[0075]
[0076] Dissolve 0.63 g of 2mmol tetrabutylammonium fluoride trihydrate into 1mL of re-evaporated dry acetonitrile, heat in a nitrogen atmosphere, and continuously feed nitrogen to evaporate the acetonitrile to dryness, then add 2mL of acetonitrile and repeat the above operation twice, and add 1mmol of the compound 10mL redistilled acetonitrile solution of B, the reaction mixture was refluxed for 6h under a nitrogen atmosphere, TLC showed that the reaction was complete, the acetonitrile was spinned off, column chromatography separation and purification can be obtained 19 F generation product, the yield is about 60%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com