Freeze-dried powder injection of tropisetron hydrochloride composition serving as vomit-stopping drug and preparation method of freeze-dried powder injection
A technology for tropisetron hydrochloride and freeze-dried powder injection, which is applied in the field of medicine, can solve the problems such as unsatisfactory hygroscopicity of impurity content crystal form, difficulty in preparation preparation, influence on stability, etc., and achieves low content of insoluble particles and good fluidity , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
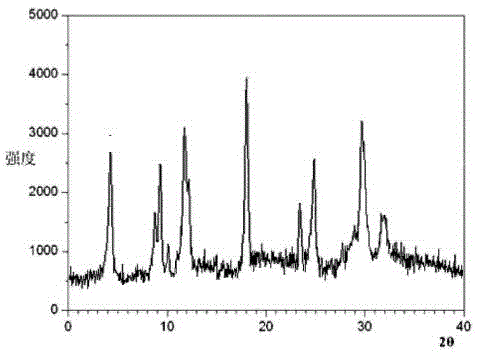
[0027] Example 1: Preparation of Tropisetron Hydrochloride Crystals
[0028] (1) Grind the crude product of tropisetron hydrochloride, pass it through a 60-mesh sieve, then add it into deionized water whose volume is 6 times the weight of tropisetron hydrochloride, and stir at 130 rpm for 10 minutes;
[0029] (2) Add ethanol whose volume is 4 times the weight of tropisetron hydrochloride under stirring at 90 rpm, and raise the temperature to 30°C at the same time;
[0030] (3) After the solution is added, let it stand for 3 hours, and add dropwise a mixed solution of ether and chloroform whose volume is 8 times the weight of tropisetron hydrochloride at 0°C under the condition of stirring at 150 rpm. The volume ratio of methane is 2: 3, and the uniform dropwise addition is completed within 2h;
[0031] (4) After the dropwise addition was completed, the temperature was lowered to -5°C, and stirring was continued at a stirring rate of 110 rpm for 2 h, and the crystals were pr...
Embodiment 2
[0033] Example 2: Preparation of tropisetron hydrochloride freeze-dried powder injection:
[0034] Prescription: in parts by weight, 3 parts of tropisetron hydrochloride crystalline compound prepared in Example 1, and 15 parts of trehalose.
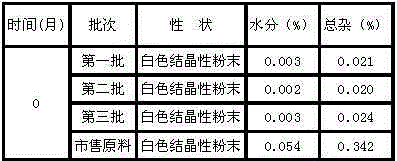
[0035] Take the tropisetron hydrochloride compound of the present invention, stir and dissolve it with water for injection, add the prescribed amount of trehalose, adjust the pH value to 6.0-8.0, then stir until the pH remains constant, add water for injection until the volume of the solution is tropane hydrochloride 100 times the weight of setron, then coarsely filter with activated carbon, pass through 1.0μm, 0.45μm, 0.22μm microporous membranes in turn to sterilize and filter, filter into a sterile room, measure pH and content after passing, fill, press half Stopper, put it into a freeze-drying box that has been cooled to -40°C, freeze-dry at low temperature, press the stopper out of the box, and roll the cap.
[0036] Described fre...
Embodiment 3
[0040] Example 3: Preparation of tropisetron hydrochloride freeze-dried powder injection:
[0041] Prescription: in parts by weight, 3 parts of tropisetron hydrochloride crystalline compound prepared in Example 1, and 18 parts of trehalose.
[0042] Take the tropisetron hydrochloride compound of the present invention, stir and dissolve it with water for injection, add the prescribed amount of trehalose, adjust the pH value to 6.0-8.0, then stir until the pH remains constant, add water for injection until the volume of the solution is tropane hydrochloride 100 times the weight of setron, then coarsely filter with activated carbon, pass through 1.0μm, 0.45μm, 0.22μm microporous membranes in turn to sterilize and filter, filter into a sterile room, measure pH and content after passing, fill, press half Stopper, put it into a freeze-drying box that has been cooled to -40°C, freeze-dry at low temperature, press the stopper out of the box, and roll the cap.
[0043] Described fre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com