Vomit-stopping drug, namely, tropisetron hydrochloride composition tablets
A technology for tropisetron hydrochloride and a composition, which is applied in the field of medicine, can solve the problems of unsatisfactory hygroscopicity of impurity content crystal form, difficult preparation of preparations, influence on stability, etc., and achieves safe and reliable clinical application, good fluidity, and dissolution. high degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
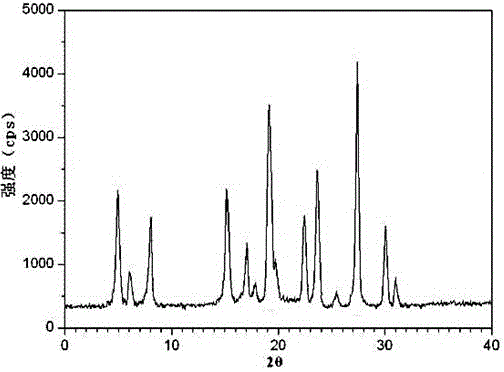
[0029] Example 1: Preparation of Tropisetron Hydrochloride Crystals
[0030] (1) Grind the crude product of tropisetron hydrochloride, pass it through a 80-mesh sieve, then add it into methanol whose volume is 11 times the weight of tropisetron hydrochloride, and stir at 130 rpm for 10 minutes;
[0031] (2) Add acetone whose volume is 6 times the weight of tropisetron hydrochloride under stirring at 90 rpm, and raise the temperature to 45°C at the same time;
[0032] (3) After adding the solution, let it stand for 2 hours, and add dropwise isobutanol at -5°C, whose volume is 8 times the weight of tropisetron hydrochloride, under the condition of stirring at 240 rpm, and finish adding dropwise at a uniform speed within 1.5 hours;
[0033] (4) After the dropwise addition was completed, the temperature was lowered to -10°C, and stirring was continued at a stirring rate of 110 rpm for 2 hours, and crystals were precipitated after standing for 2 hours, filtered, washed, and vacuu...
Embodiment 2
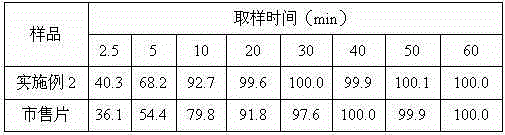
[0035] Example 2: Preparation of Tropisetron Hydrochloride Tablets
[0036] Prescription: 0.5 parts by weight of tropisetron hydrochloride crystal form compound obtained in Example 1, 10 parts by weight of starch, 4 parts by weight of magnesium aluminum silicate, 2.5 parts by weight of crospovidone, 0.25 parts by weight of povidone K300. , 0.08 parts by weight of sodium dodecylbenzenesulfonate, 2.5 parts by weight of purified water, 0.35 parts by weight of magnesium stearate;
[0037] Preparation:
[0038] (1) Weighing: Weighing according to the process prescription;
[0039] (2) Treatment of raw and auxiliary materials: Mix the prescribed amount of tropisetron hydrochloride and the prescribed amount of magnesium aluminum silicate in equal increments, mix evenly, and pulverize through a 100-mesh sieve to obtain a mixture of raw and auxiliary materials;
[0040] (3) Preparation of adhesive: Take the prescribed amount of purified water and place it in a stainless steel bucke...
Embodiment 3
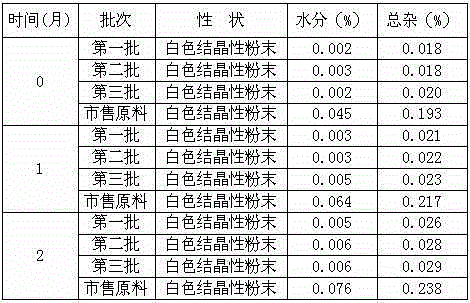
[0046] Example 3: Preparation of Tropisetron Hydrochloride Tablets
[0047] Prescription: 0.5 parts by weight of tropisetron hydrochloride crystal compound obtained in Example 1, 12 parts by weight of starch, 4.2 parts by weight of magnesium aluminum silicate, 3 parts by weight of crospovidone, and 300.3 parts by weight of povidone K , 0.1 parts by weight of sodium dodecylbenzenesulfonate, 3 parts by weight of purified water, 0.4 parts by weight of magnesium stearate;
[0048] Preparation:
[0049] (1) Weighing: Weighing according to the process prescription;
[0050] (2) Treatment of raw and auxiliary materials: Mix the prescribed amount of tropisetron hydrochloride and the prescribed amount of magnesium aluminum silicate in equal increments, mix evenly, and pulverize through a 100-mesh sieve to obtain a mixture of raw and auxiliary materials;
[0051] (3) Preparation of adhesive: Take the prescribed amount of purified water and put it in a stainless steel bucket, add the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com