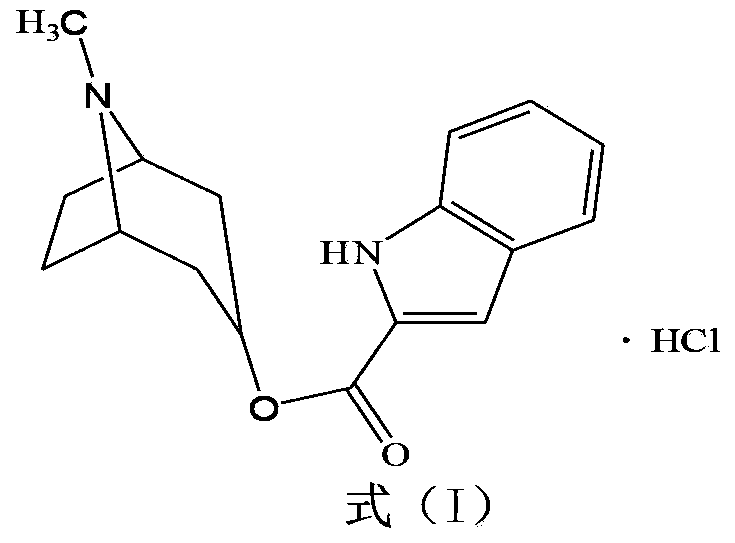
Tropisetron hydrochloride compound, its preparation method, and pharmaceutical composition containing the same
A technology of tropisetron hydrochloride and compounds, which is applied in the field of medicine, can solve problems such as unsatisfactory fluidity, and achieve the effects of improving fluidity, good stability, and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] [Example 1] Preparation of Tropisetron Hydrochloride Compound
[0053] 1) Take 50g of tropisetron hydrochloride raw material, add 150ml of mixed solvent of ethanol and tetrahydrofuran (the volume ratio of ethanol and tetrahydrofuran is 3:1), heat to 50°C, keep it warm for 30 minutes, filter while it is hot, and obtain the filtrate for later use ;
[0054] 2) Naturally cool the spare filtrate to room temperature;
[0055] 3) Add 30ml of ethyl acetate dropwise to the filtrate at a stirring speed of 20rpm, continue stirring for 30 minutes after dropping, let stand for 8 hours, filter to obtain a filter cake, wash the filter cake with ethanol, and then dry it under reduced pressure for 2 hours , to obtain the tropisetron hydrochloride compound. Yield 71.2%, HPLC content 99.95%.
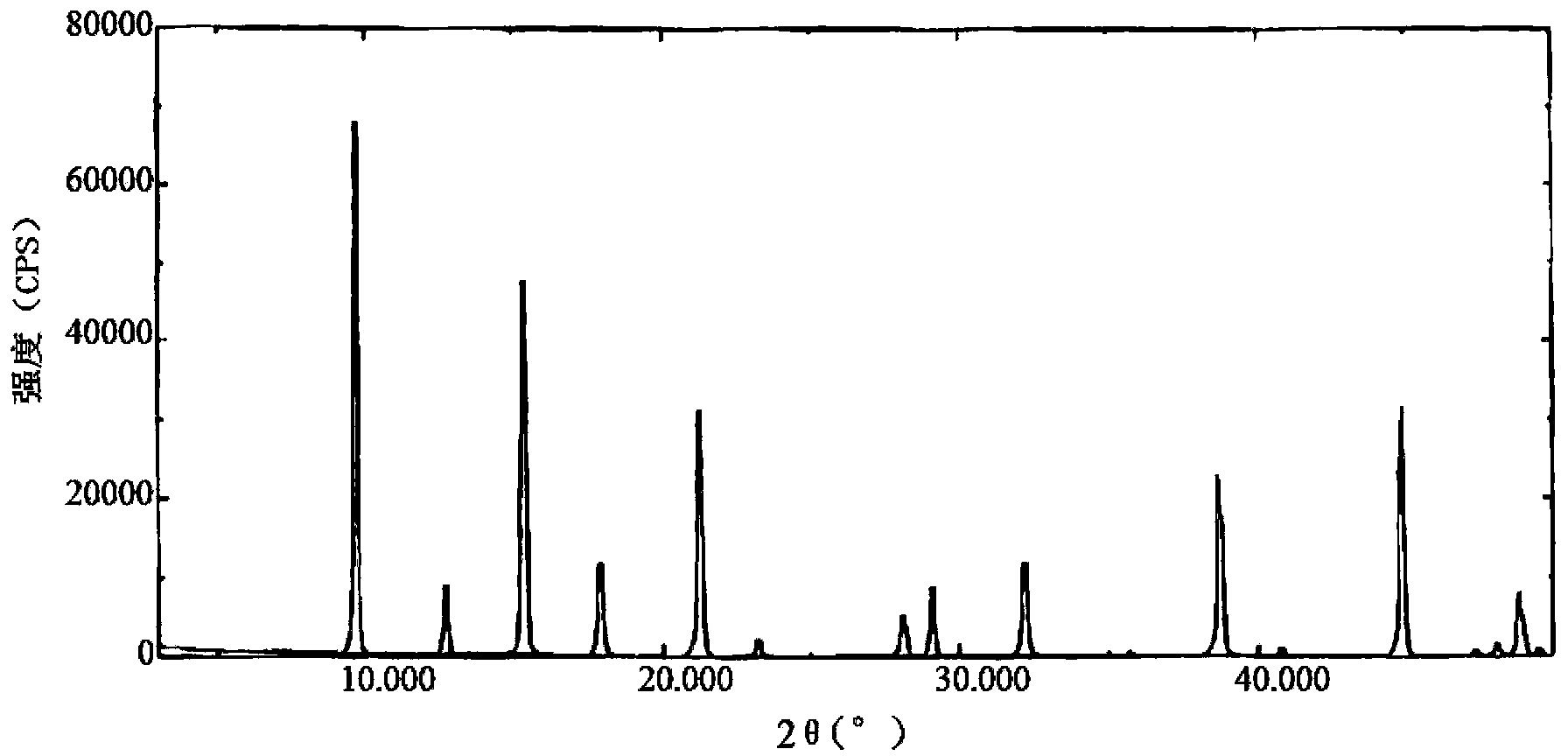
[0056] The X-ray powder diffraction pattern of the tropisetron hydrochloride compound of gained is shown in figure 1 shown. It is measured by D / Max-2500.9161 X-ray diffractometer, measurement ...
Embodiment 2
[0057] [embodiment 2] preparation of tropisetron hydrochloride compound
[0058] 1) Take 50g of tropisetron hydrochloride raw material, add 350ml of mixed solvent of ethanol and tetrahydrofuran (the volume ratio of ethanol and tetrahydrofuran is 8:1), heat to 60°C, keep it warm for 15 minutes, filter while it is hot, and obtain the filtrate for later use ;
[0059] 2) Naturally cool the spare filtrate to room temperature;
[0060] 3) Add 43.75ml of ethyl acetate dropwise to the filtrate at a stirring speed of 30rpm, continue stirring for 20 minutes after dropping, let stand for 6 hours, filter to obtain a filter cake, wash the filter cake with ethanol, and then dry under reduced pressure 4h to obtain the tropisetron hydrochloride compound. Yield 71.6%, HPLC content 99.98%.
[0061] The X-ray powder diffraction pattern of the obtained tropisetron hydrochloride compound is consistent with the results of Example 1, and its assay method is the same as that of Example 1.
Embodiment 3
[0062] [embodiment 3] preparation of tropisetron hydrochloride compound
[0063] 1) Take 50g of tropisetron hydrochloride raw material, add 210ml of mixed solvent of ethanol and tetrahydrofuran (the volume ratio of ethanol and tetrahydrofuran is 6:1), heat to 55°C, keep it warm for 20 minutes, filter while it is hot, and obtain the filtrate for later use ;
[0064] 2) Naturally cool the spare filtrate to room temperature;
[0065] 3) Add 30ml of ethyl acetate dropwise to the filtrate at a stirring speed of 25rpm. After dropping, continue to stir for 25 minutes, let it stand for 7 hours, and filter to obtain a filter cake. The filter cake is washed with ethanol, and then dried under reduced pressure for 3 hours , to obtain the tropisetron hydrochloride compound. Yield 71.3%, HPLC content 99.96%.
[0066] The X-ray powder diffraction pattern of the obtained tropisetron hydrochloride compound is consistent with the results of Example 1, and its assay method is the same as that...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com