Triopisetron Hydrochloride microcapsule and method for preparing injection
A technology for tropisetron hydrochloride and injection, which is applied in capsule delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc. It can solve the problems of poor clarity, hydrolysis and separation of tropisetron hydrochloride, etc., and achieve slow release , Improve bioavailability, improve the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
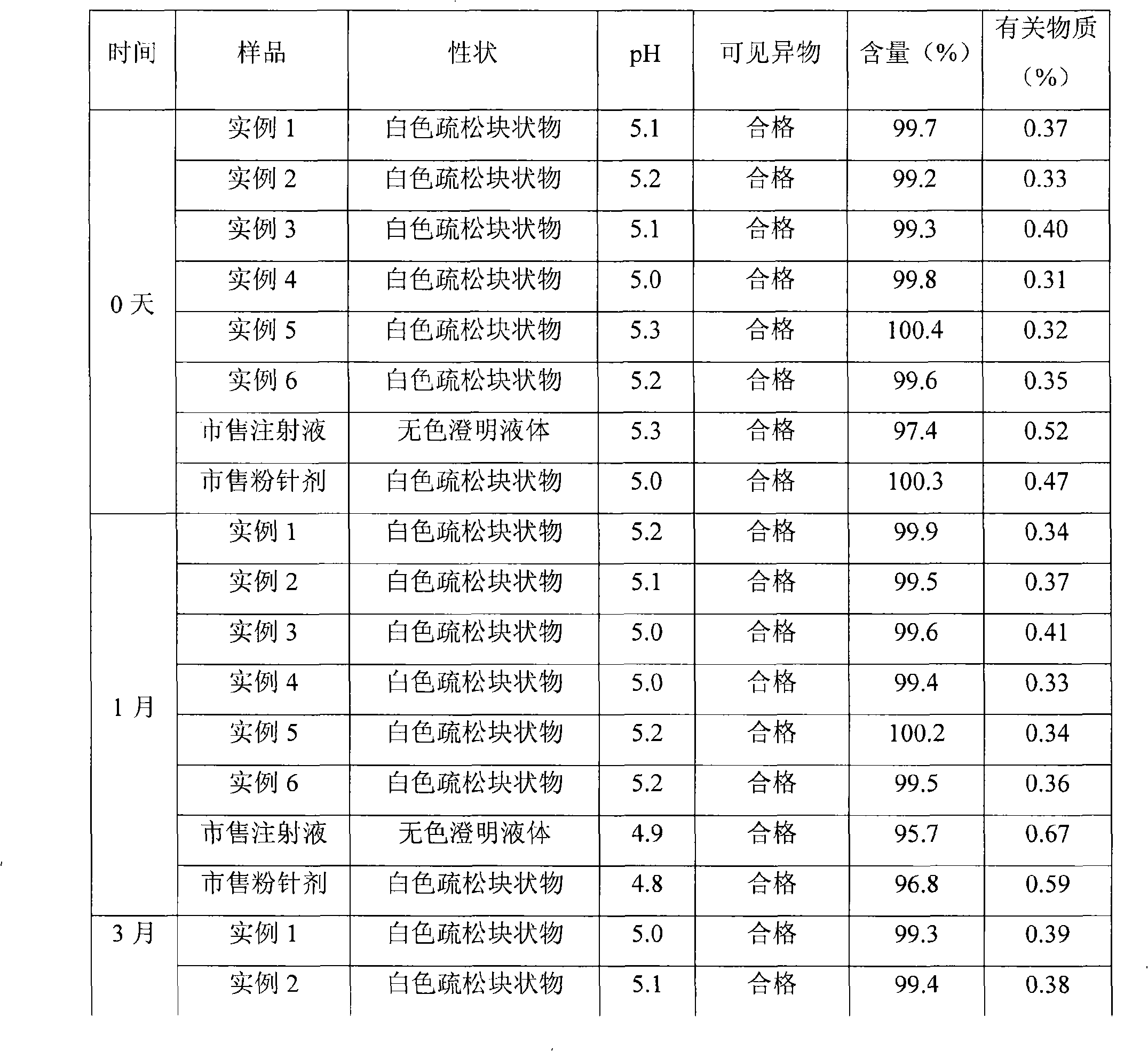
Examples
Embodiment 1
[0061] Tropisetron Hydrochloride 2g
[0062] Gelatin 6g
[0063] Dextran 5g
[0064] Soy Lecithin 1.1g
[0065] Tween 80 1.5g
[0066] Mannitol 50g
[0067] Preparation Process
[0068] (1) 2g tropisetron hydrochloride is added in the water for injection, dissolves completely;
[0069] (2) Add 6g gelatin and 1.1g soybean lecithin to the solution obtained in step (1), prepare water-in-oil emulsion with mechanical stirring;
[0070] (3) Add 200ml of 2.5% dextran solution and 1.5g Tween 80 to the emulsion obtained in step (2), and prepare a water-in-oil-in-water multiple emulsion by mechanical stirring;
[0071] (4) The multiple emulsion obtained in step (3) is dripped into the 2mol / L sodium hydroxide solidified liquid in the high-voltage electrostatic field by an orifice injection, the electric field voltage is 2.2kv, and the injection speed is 15ml / min, after solidifying for 20min Filter out the microcapsules, inject the double emulsion, and then stir the strong alkali c...
Embodiment 2
[0074] Tropisetron Hydrochloride 5g
[0075] Gelatin 20g
[0076] Dextran 8g
[0078] Poloxamer 188 7.0g
[0079] Glucose 300g
[0080] The preparation process is the same as in Example 1, and tropisetron hydrochloride freeze-dried powder injection is obtained.
Embodiment 3
[0082] Tropisetron Hydrochloride 3g
[0083] Gelatin 10g
[0084] Dextran 5g
[0085] Cholesterol 2.7g
[0086] Poloxamer 188 3.6g
[0087] Glucose 120g
[0088] The preparation process is the same as in Example 1, and tropisetron hydrochloride freeze-dried powder injection is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com