Antitumor vaccination using allogeneic tumor cells expressing alpha (1,3)-galactosyltransferase
A tumor cell, galactose-based technology, applied in the direction of anti-tumor drugs, carrier-bound antigen/hapten components, antibodies, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
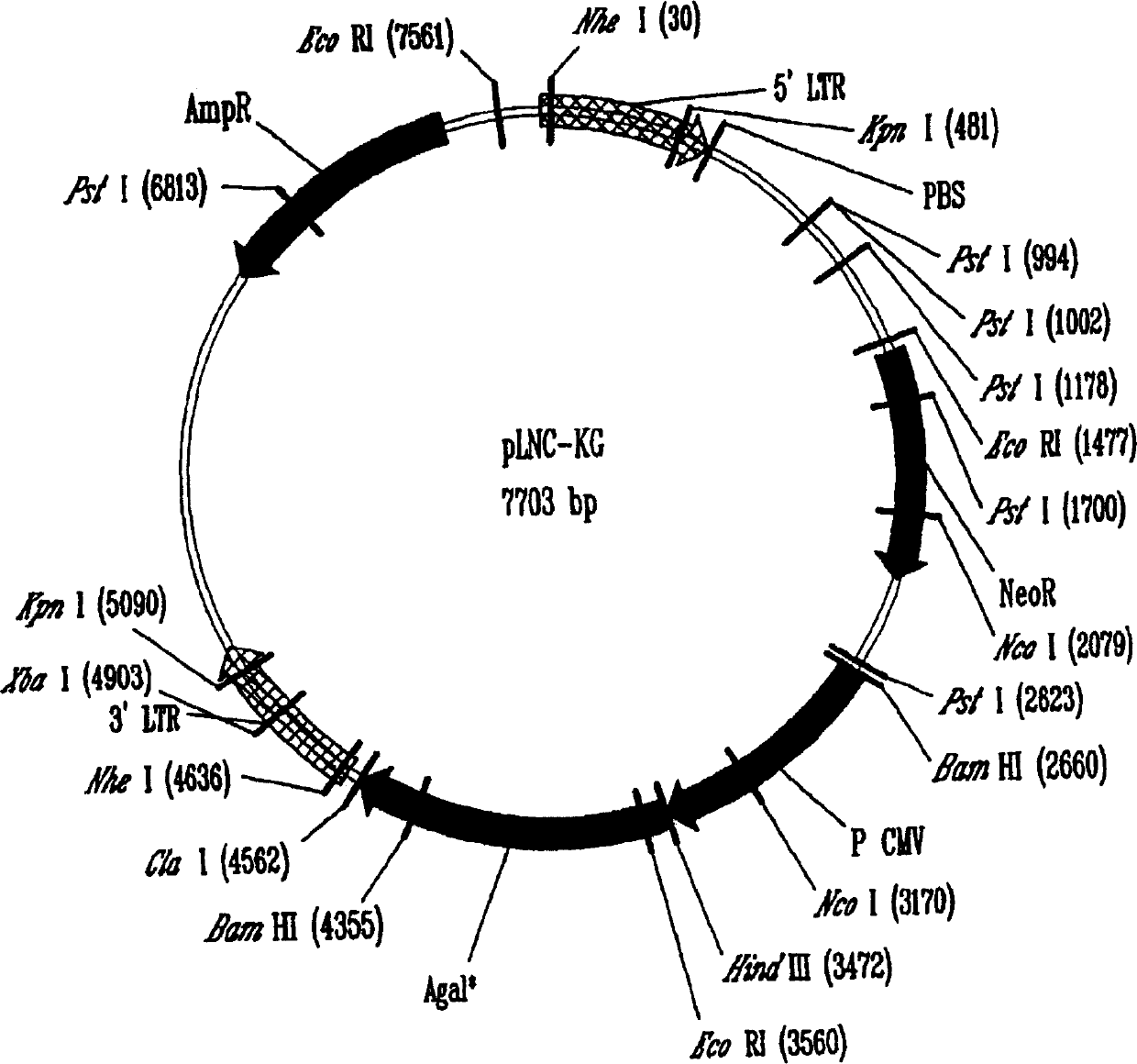
[0146] Generation of a retroviral vector expressing αGT, pLNCKG
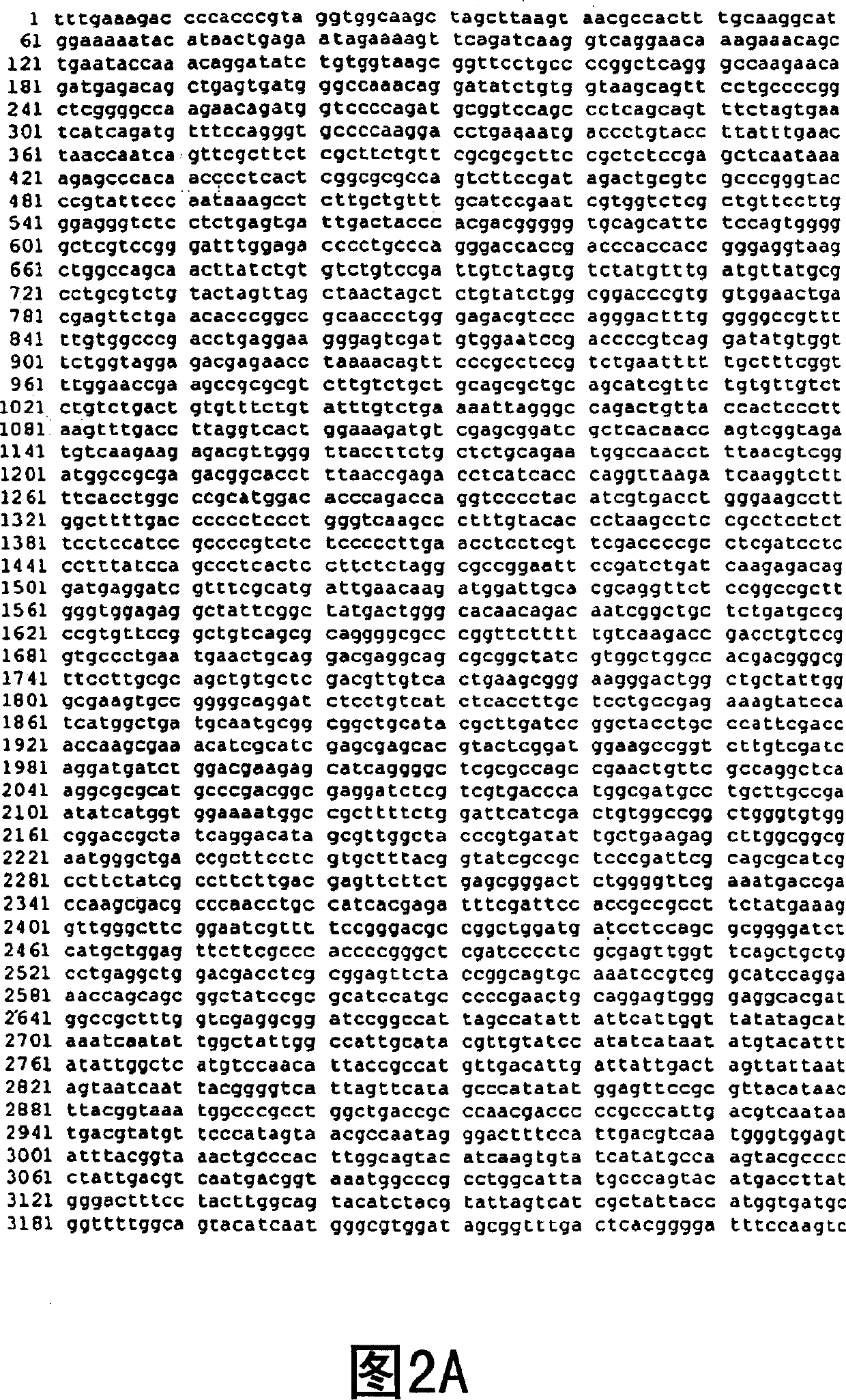
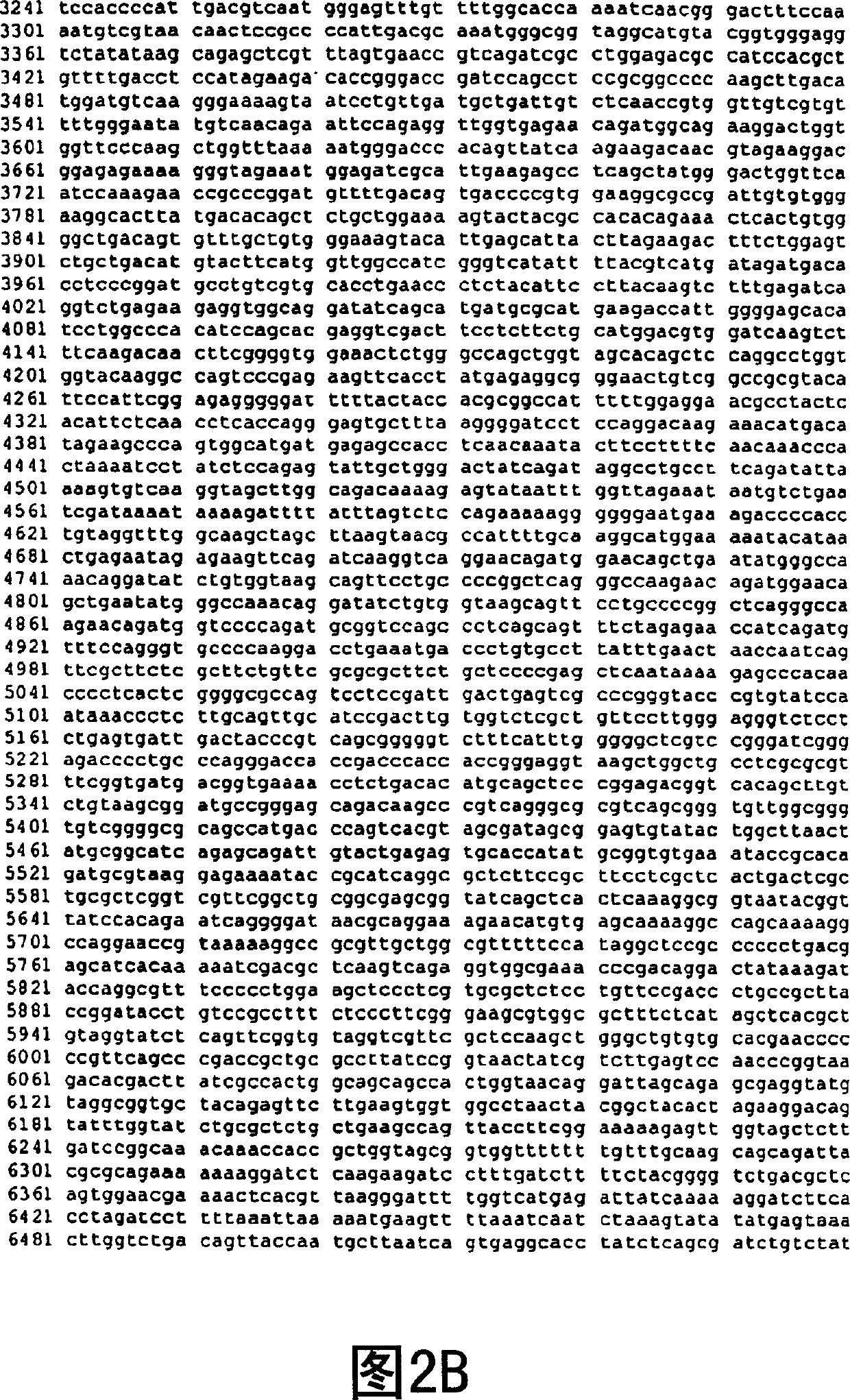
[0147] Using the forward primer 5′-ACAAAAGCTTGACATGGATGTCAAGGGAAAAGTAAT-3′ and the reverse primer 5′-AATTATCGATTCAGACATTATTTCTAAC-3′, a 1077 bp fragment of the murine αGT gene containing the Kozak sequence that enhances αGT translation was amplified by PCR and cloned into pLNCX ClaI and HindIII sites to generate the pLNCKG retroviral vector (Figure 1). This vector was transfected into the packaging cell line 293. AMIZ [Young and Link "Chimericretroviral helper virus and picornavirns IRES sequence to eliminate DNAmethylation for improved retroviral packaging cells" J. Virol. (2000) 74:5242-5249] to generate vector production cells Department 293.AMIZ / LNCKG. In the presence of G418 and Zeocin TM Case selection for transfected cells for two weeks. Mixed selection cell populations were subcloned by limiting dilution. Single cell-derived VPCs were screened for their ability to efficiently transduce human epitheli...
Embodiment 2
[0149] Transduction of B16.BL6 melanoma cells with LNCKG retroviral vector
[0150] To produce αGal (+) For B16 cells, use 2 mL containing 2 x 10 6 The supernatant of the LNCKG retrovirus at tu / mL infection titer was transduced 2×10 6 cell. Cells were selected for neomycin resistance by two weeks of selection in medium supplemented with G418 1 mg / mL. Following this selection, cells were stained with chicken anti-αGal polyclonal antibody for expressed αGal epitopes and sorted by fluorescence activated cell sorting.
Embodiment 3
[0152] Anti-αGal antibodies were induced in α(1,3)galactosyltransferase knockout (αGT KO) mice by immunization with rabbit erythrocytes.
[0153] Female and male 8-14 week old α(1,3)galactosyltransferase (αGT) knockout (KO) mice were used in this study. The mice were initially mixed haplotype (H-2 b / d) and the current colony of αGT KO mice was obtained by breeding and selection in the F4 cross generation of the H-2 b / b haplotype. ). These animals produce low titers of native antibodies against the [alpha]Gal epitope. To increase the titer of anti-αGal Ab, mice were treated with 1×10 8 Rabbit erythrocytes were immunized intraperitoneally twice with an interval of two weeks. Anti-αGal Ab titers were tested one week after the last RRBC injection to confirm that all mice in this study had high titers of anti-αGal Ab. All mice used in this study had high anti-αGal Ab above a 1 :500 dilution as measured by ELISA. A representative experiment is shown in Figure 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com