Vaccine and preparation method thereof
A vaccine and antigen technology, applied in the biological field, to achieve the effects of high safety, huge market value, and quick and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
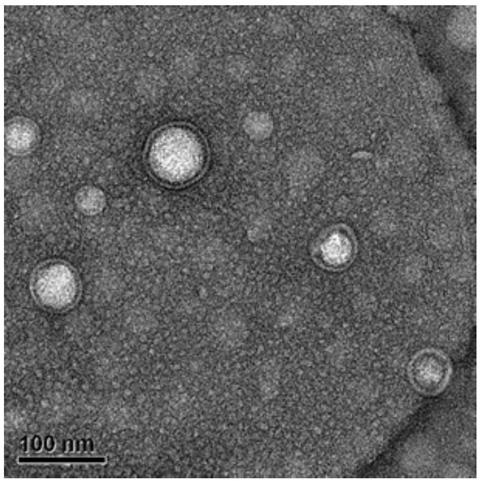
[0053] Inoculate the DH5-α strain in the corresponding culture medium, culture overnight at 37°C, 220rpm; use a centrifuge at 6000×g, centrifuge at 4 degrees for 20min, pass the supernatant of the bacterial liquid through a 0.45μm filter membrane, and concentrate the filtrate to 100kDa ultrafiltration tube 100mL, the concentrated product was passed through a 0.22μm filter membrane to filter out other cells and debris, and the obtained filtrate was ultracentrifuged at 4 degrees, 150000×g for 3h, and the obtained OMVs were resuspended in PBS, and the protein concentration was detected by BCA;
[0054] Connect 4 arginines to the N-terminal of the tumor-specific antigen TRP2 antigen peptide to make it positively charged, dissolve in dimethyl sulfoxide solvent at room temperature; mix OMV and antigen peptide according to 1:1, and store on ice Sonicate for 30s under bath conditions, dilute to an appropriate concentration with PBS, observe the morphology and particle size with a biolo...
Embodiment 2
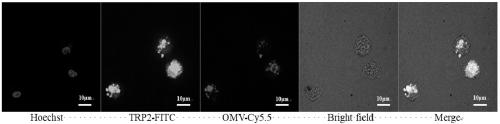
[0056] Add excess cy5.5-N-hydroxysuccinimide ester to the OMV solution and react overnight at 4°C to prepare OMV-cy5.5; modify the N-terminus of the antigen peptide with FITC fluorescent molecules (modified TRP2 antigen is used in this example Peptide, positively charged), using OMV-Cy5.5 and TRP2-FITC to prepare anti-tumor vaccine, culture BMDC cells, connect 0.4milliomBMDC to each dish with confocal glass bottom dish, add equivalent of 10μg OMV anti-tumor vaccine, and incubate for 24h;
[0057] The nuclei were stained with Hoechst33342 by 1000-fold dilution for 5 minutes, washed three times with PBS, then fixed with tissue fixative for 15 minutes, washed three times with PBS, and finally detected with a single-photon laser confocal imaging system. The results are shown in figure 2 ,Depend on figure 2 The uptake of anti-tumor vaccine by BMDC cells can be known.
Embodiment 3
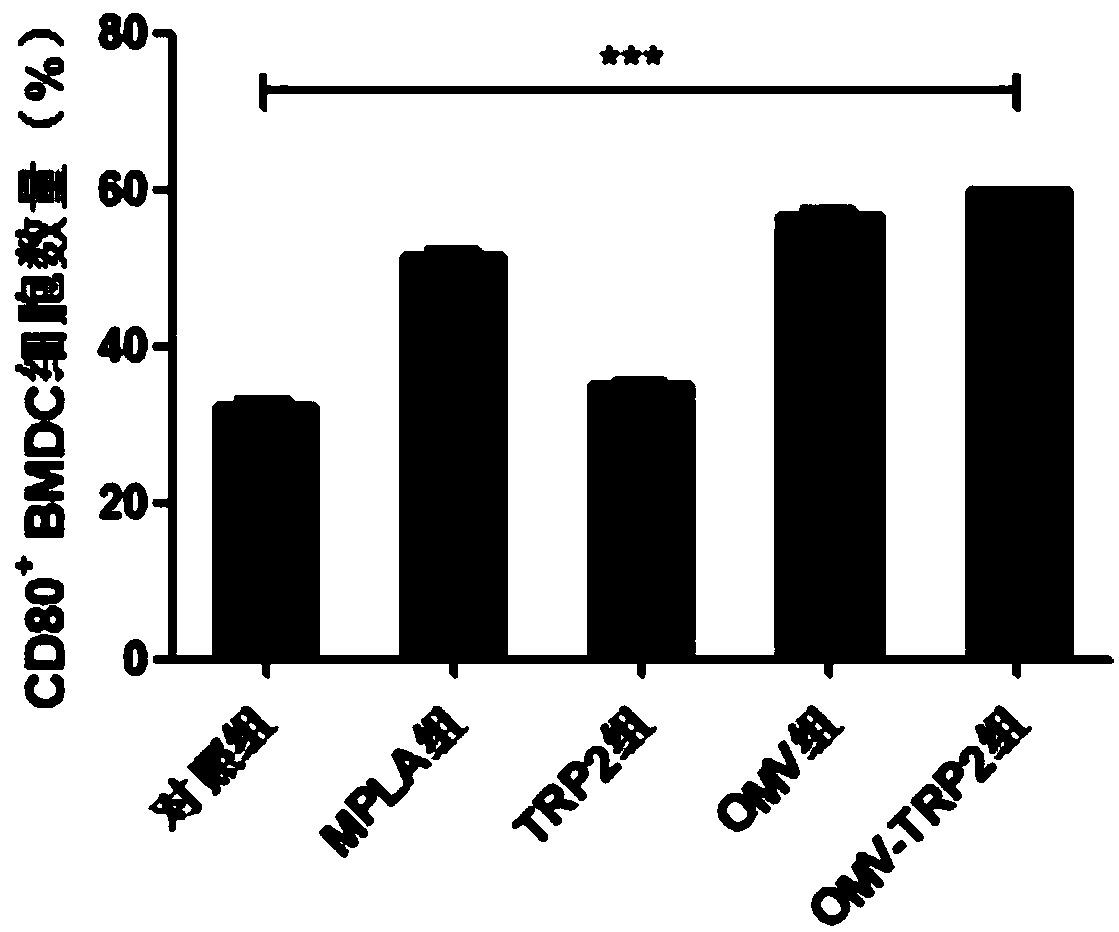
[0059] Culture BMDC cells, be divided into 5 groups, be respectively Control group, MPLA positive control group, tumor-specific antigen group (use TRP2 in this example), OMV group, antitumor vaccine group (use OMV-TRP2 in this example), each Group about 40,000 cells, and the materials added are equivalent to 10 μg OMV;
[0060] After co-incubating the material with the cells for 24 hours, collect the cells by centrifugation at 1000 rpm for 5 minutes, dilute PE anti-mouse CD11c antibody and FITC anti-mouse CD80 antibody 100 times with FACSBuffer, prepare antibody staining solution, add 50 μL to each group of cells, 4 Stain in the dark at ℃ for at least 20 minutes, add 1 mL FACS Buffer, centrifuge at 2000 rpm for 5 minutes, discard the supernatant, shake and resuspend, and analyze with flow cytometry. Similarly, use the same method to detect CD86 and evaluate the maturation of BMDC. The results are shown in Figure 3(A) and Figure 3(B) reflect the maturation of BMDC cells after b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com