Recombinant plasmid, recombinant malaria parasite and its application
A technology of recombining plasmids and malaria parasites, which is applied in the field of tumor cell immunotherapy, and can solve problems such as the inability to express multiple foreign genes at the same time, the inability to secrete genes, and the reverse insertion of target genes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
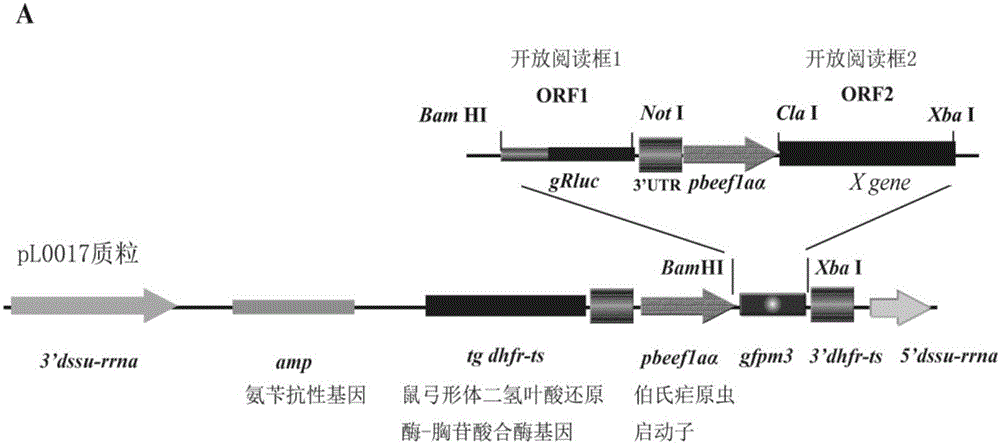
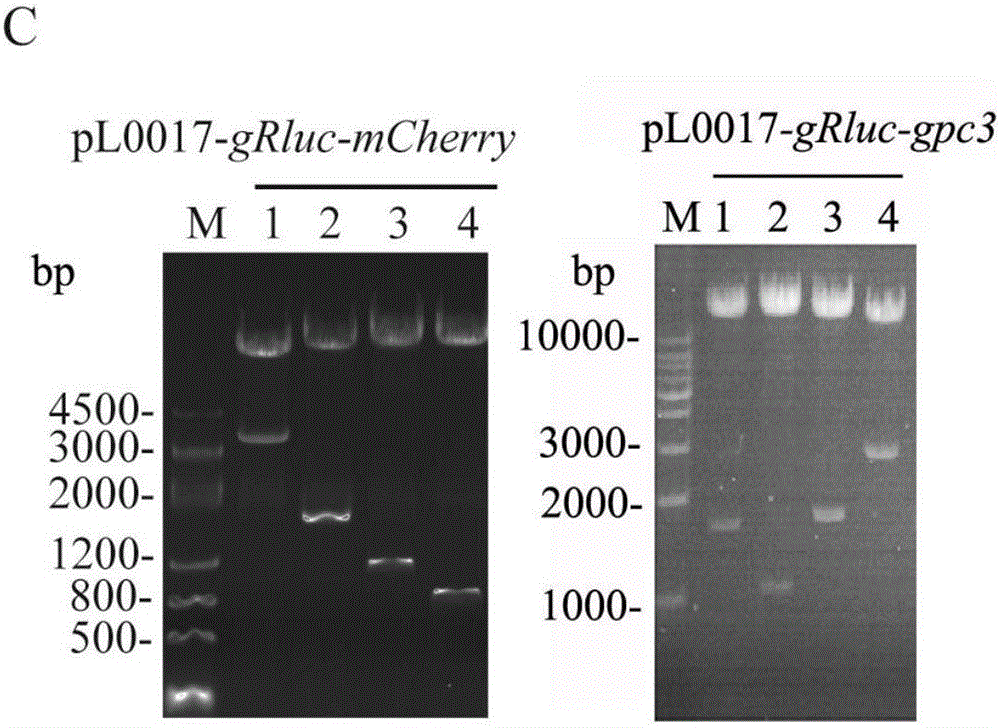
[0057] Example 1 Preparation of recombinant plasmid pL0017-gRluc-X
[0058] 1. The basic molecular cloning technology used in this experiment:
[0059] 1) Extraction of plasmids.
[0060] Refer to the instructions of AxyPrep Plasmid DNA Mini Kit from Axygen Company (Axygen, #AP-MN-P-50).
[0061] 2) Gel DNA fragment extraction.
[0062] Follow the instructions of AxyPrep DNA Gel Recovery Kit from Axygen Company.
[0063] 3) Enzyme digestion of DNA and carrier identification.
[0064] The plasmid DNA was prepared into a suitable reaction system with the corresponding restriction endonuclease and its supporting buffer, and was bathed in 37°C water bath for 3-4 hours to identify the result of enzyme digestion.
[0065] The reaction system for enzyme digestion and recovery of DNA is as follows: 3 μl of 10× buffer, 20 μl of enzyme digestion carrier DNA, 0.5 μl of each enzyme E1 / E2 (choose a restriction enzyme according to the purpose of the experiment), 6 μl of ddH 2 O, the to...
Embodiment 2
[0114] The construction of embodiment 2 recombinant malaria parasites
[0115] 1. Large-dose extraction of pL0017-gRluc-mCherry plasmid, pL0017-gRluc-ibisi-mCherry plasmid, pL0017-gRluc-gpc3 plasmid, pL0017-gpc3:2F plasmid and pL0017-ibis1-gpc3:2F plasmid.
[0116] 1) Plasmid extraction
[0117] (1) Streak activation contains correctly constructed recombinant pL0017 plasmids, including pL0017-gRluc-mCherry plasmid, pL0017-gRluc-ibisi-mCherry plasmid, pL0017-gRluc-gpc3 plasmid, pL0017-gpc3:2F plasmid and pL0017-ibis1-gpc3 : the bacterial strain of 2F plasmid, culture 16h in the incubator;
[0118] (2) Randomly pick 2 single clones, transfer them to a 15ml centrifuge tube and culture them for 8-12 hours;
[0119] (3) According to the inoculum size of 1:500, inoculate the above-mentioned culture solution into a 1-liter Erlenmeyer flask containing 200 ml of ampicillin-resistant LB medium, shake and culture at 37°C for 12 hours, and collect the bacterial solution with QIAGEN’s la...
Embodiment 3
[0187] Embodiment 3 Immunological detection of murine Plasmodium yoelii immunized mice
[0188] Including CD8α+DC, Th1-related cytokines, and CTL response detection against GPC3 protein.
[0189] 1. Experimental materials: C57BL / 6 mice, Hepa1-6 cells; penicillin and streptomycin are products of Bio Basic Inc; pancreatin is a product of Amresco. Anti-CD11c-FITC, anti-CD8α-PE, anti-CD86-APC, anti-CD80-PerCP-Cy5.5 antibodies (purchased from eBioscience, San Diego, CA, USA), BD FACSAria flow cytometer, FlowJo analysis software (Tree Star , Inc.), mouse Th-related multifactor detection kit (BioLegend), BDTM ELISPOT MouseIFN-γ-Kit (BD Biosciences, #552569). Fortessa flow analyzer (BD), Legendplex analysis software (BioLegend); 1 × erythrocyte lysate (BD), 200 mesh cell filter, 1mL syringe, low-temperature refrigerated centrifuge (Germany eppendorf company), biochemical incubator (Shanghai No. Heng Instrument Company), tweezers, scissors, alcohol, capillary glass tube; DMEM and RPM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com