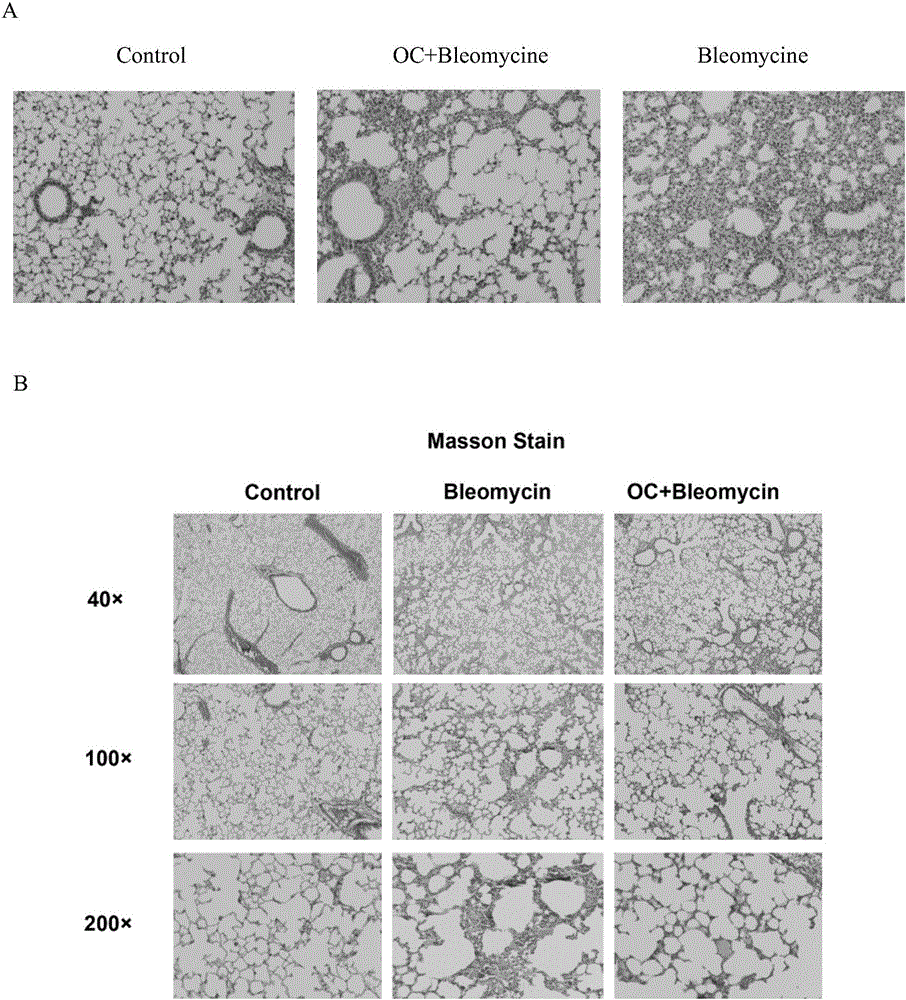
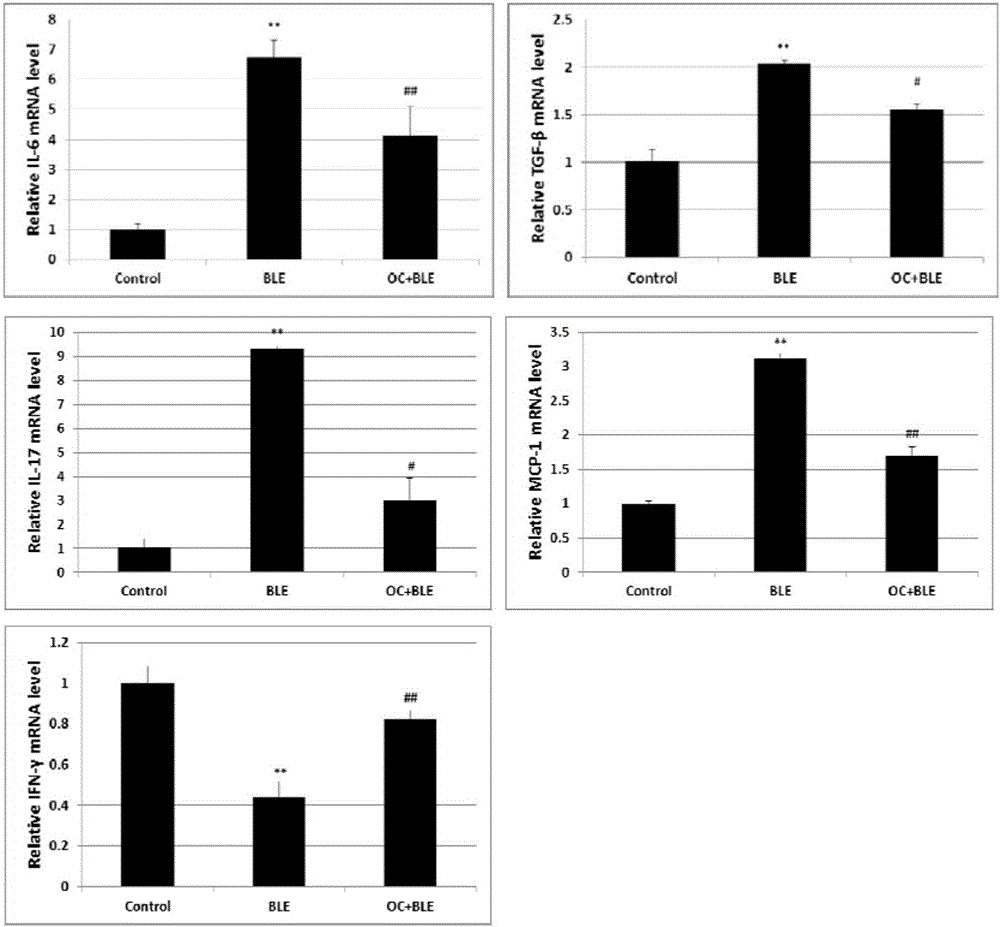
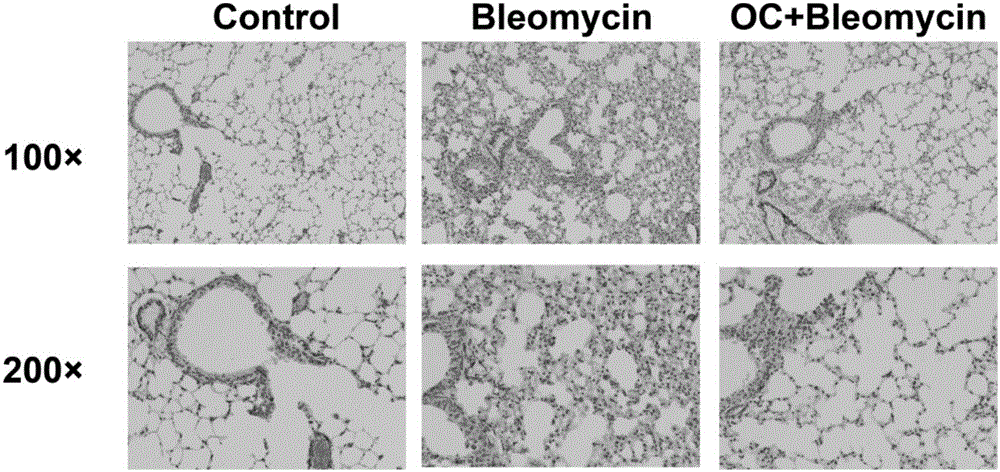
Application of obakunone to preparation of medicine for preventing and treating lung injury and pulmonary fibrosis
A technology of pulmonary fibrosis, cortex ketone, applied in the active field of anti-lung injury and anti-pulmonary fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0060] Example 1 (injection)
[0061] 1. Prescription:
[0062]
[0063] 2. Preparation method
[0064] Soak phellodendron in soybean oil and oleic acid as the oil phase, stir continuously and heat to 70°C, disperse polyethylene glycol 15-hydroxystearate, soybean lecithin, glycerin, and hexasodium phytate in water as the water phase , continuously stirring and heating to 70°C, slowly adding the oil phase to the water phase, stirring at a high speed with a high-speed disperser for 8 minutes (rotating speed 2000 rpm) to obtain colostrum, and homogenizing the colostrum with a high-pressure homogenizer (pressure 65MPa , temperature 75°C) after 7 times, diluted with water for injection to 1000ml, filtered through a 0.45μm microporous membrane, filled with nitrogen, packed and melted, and autoclaved (121°C, 15min). 1ml per tube, intramuscular injection, once a day, 2 tubes each time, 10 to 20 days as a course of treatment, can be used continuously for 2 to 3 courses of treatmen...
example 2
[0065] Example 2 (tablet)
[0066] 1. Prescription:
[0067]
[0068] 2. Preparation method:
[0069] Dissolve phellodendron and hydroxypropyl cellulose SSL in 800mL of ethanol, add the above solution into liquid paraffin, dry under reduced pressure to remove ethanol, phellodendron and hydroxypropyl cellulose form fine particles to precipitate, and dry the precipitated particles at 50°C The dried microparticles are mixed evenly with croscarmellose sodium and magnesium stearate crossing 100 mesh sieves, and compressed into 10000 tablets each containing phellodendron 30mg, each with a net weight of 0.13g. Oral 2 tablets each time, once a day, 10-20 days as a course of treatment, can be used continuously for 2-3 courses.
example 3
[0070] Example 3 (capsules)
[0071] 1. Prescription:
[0072]
[0073] 2. Preparation method:
[0074] Take by weighing sodium lauryl sulfate and povidone K30 of recipe quantity, add water and dissolve, be mixed with the solution of 10% povidone K30, make adhesive; Add binders to make soft materials, and then use a granulator to granulate; dry the wet granules at 40°C to 45°C for 1 to 3 hours, and then granulate; put the above granules in a mixer, and add low-substituted Hydroxypropyl cellulose, crospovidone and magnesium stearate were mixed for 30 minutes, and samples were taken to determine content and loss on drying; the average loading was calculated according to the results of particle content measurement, and filled with 4# capsules to make 1000 capsules , each capsule is filled with 0.1g granules, containing 20mg of cortex ketone, orally 3 capsules each time, once a day, 10-20 days as a course of treatment, and can be used continuously.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com