Heparin-binding protein assay kit as well as preparation method and assay method thereof
A heparin-binding protein and kit technology, applied in the field of immunoassay, can solve the problems of lack of high-tech, the sensitivity and specificity of detection results are not ideal and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Embodiment 1 A kind of heparin-binding protein assay kit
[0090] A heparin-binding protein assay kit, comprising magnetic particles, HBP antibody 1, enzyme-labeled HBP antibody 2, HBP calibrator, concentrated washing solution and substrate.
[0091] Magnetic particles: avidin-labeled magnetic particles with a particle size of 0.9 μm to 1.1 μm;
[0092] HBP antibody 1: biotin-labeled mouse anti-human HBP monoclonal antibody 1;
[0093] Enzyme-labeled HBP antibody 2: horseradish peroxidase-labeled modified mouse anti-human HBP monoclonal antibody 2;
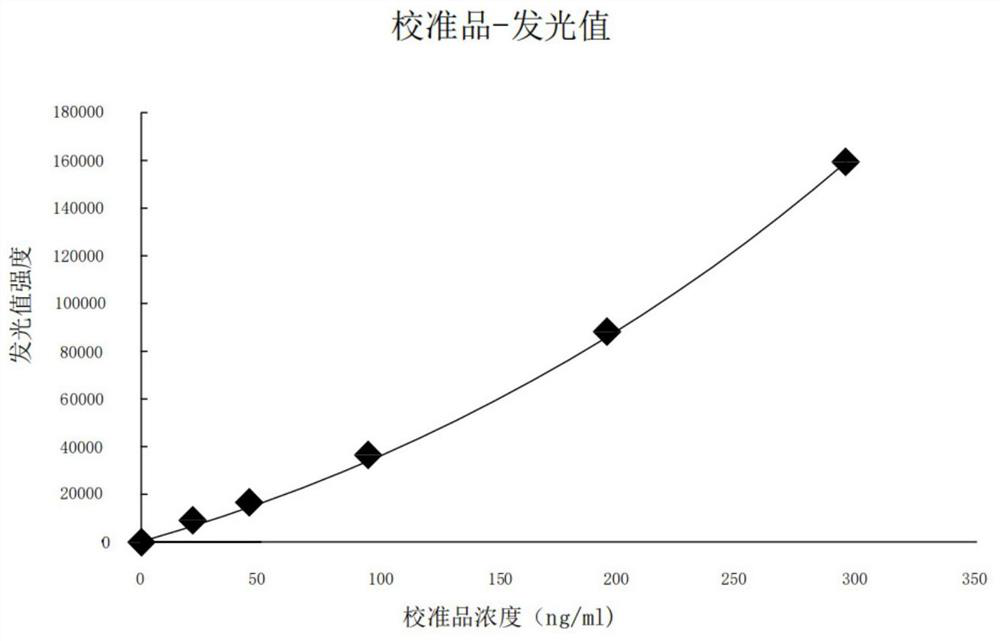
[0094] HBP calibrator: recombinant human HBP;
[0095] Concentrated washing solution: 2mol / L, pH7.5 phosphate buffer solution with mass fraction of 2% Tween-20 and 1% Procline300.
[0096] Substrate: luminol at a concentration of 1 mmol / L.
Embodiment 2
[0097] Embodiment 2 Heparin-binding protein assay kit preparation method
[0098] Step 1, preparation of avidin-labeled magnetic particles:
[0099] Activate the magnetic particles with EDC and NHS, mix them with avidin, rotate at room temperature for 2 hours, and block with blocking solution for 1 hour. The blocking solution is 1mol / L glycine, and perform magnetic separation. After removing the blocking solution, add pH 7.4, 0.02mol / L phosphate buffer containing 0.5% Tween-20, 1% BSA, 1% EDTA, 0.1% Procline300, 4% PEG for storage, and store at 2°C to 8°C for later use;
[0100] Step 2, prepare biotin-labeled mouse anti-human HBP monoclonal antibody 1:
[0101] Biotin was dissolved in phosphate buffer, the phosphate buffer was 0.02mol / L anhydrous disodium hydrogen phosphate, sodium dihydrogen phosphate solution, mixed with HBP antibody 1 and rotated at room temperature for 1 hour; the mixed solution was passed through Chromatography column to collect biotin-labeled HBP antib...
Embodiment 3
[0110] Embodiment 3 heparin-binding protein assay kit assay method
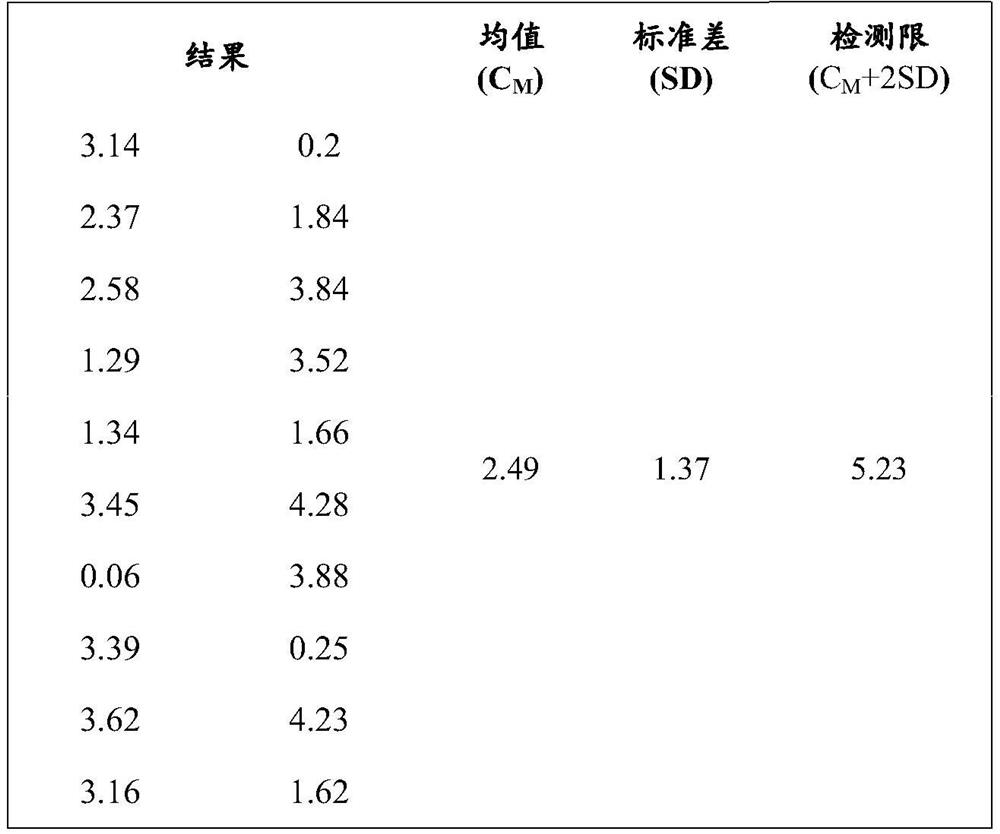
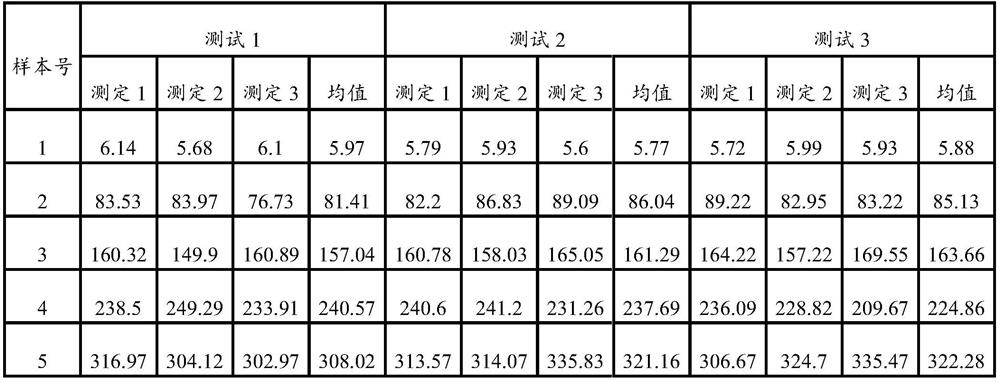
[0111]Step 1: Equilibrate the HBP calibrator, magnetic particle suspension, biotin-labeled antibody, enzyme-labeled antibody, concentrated washing solution and substrate to room temperature (20°C to 25°C);
[0112] Step 2: Dilute the concentrated lotion 10 times with purified water to become a washing working solution for later use;
[0113] Step 3: Add 50 μL of the HBP calibrator and 50 μL of the sample to be tested into 50 μL of the magnetic particle suspension in turn, add 50 μL of the biotin-labeled antibody and 50 μL of the enzyme-labeled antibody, and shake at 20°C to 25°C Incubate for 18 minutes and wash 5 times with the washing working solution; the concentration of the magnetic particles is 1 mg / ml, the concentration of the biotin-labeled antibody is 5 ug / ml, and the concentration of the enzyme-labeled antibody is 5 ug / ml;
[0114] Step 4: Add 50 μL of water and mix well;
[0115] Step 5: add 100 μ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Linear range | aaaaa | aaaaa |
| Linear | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com