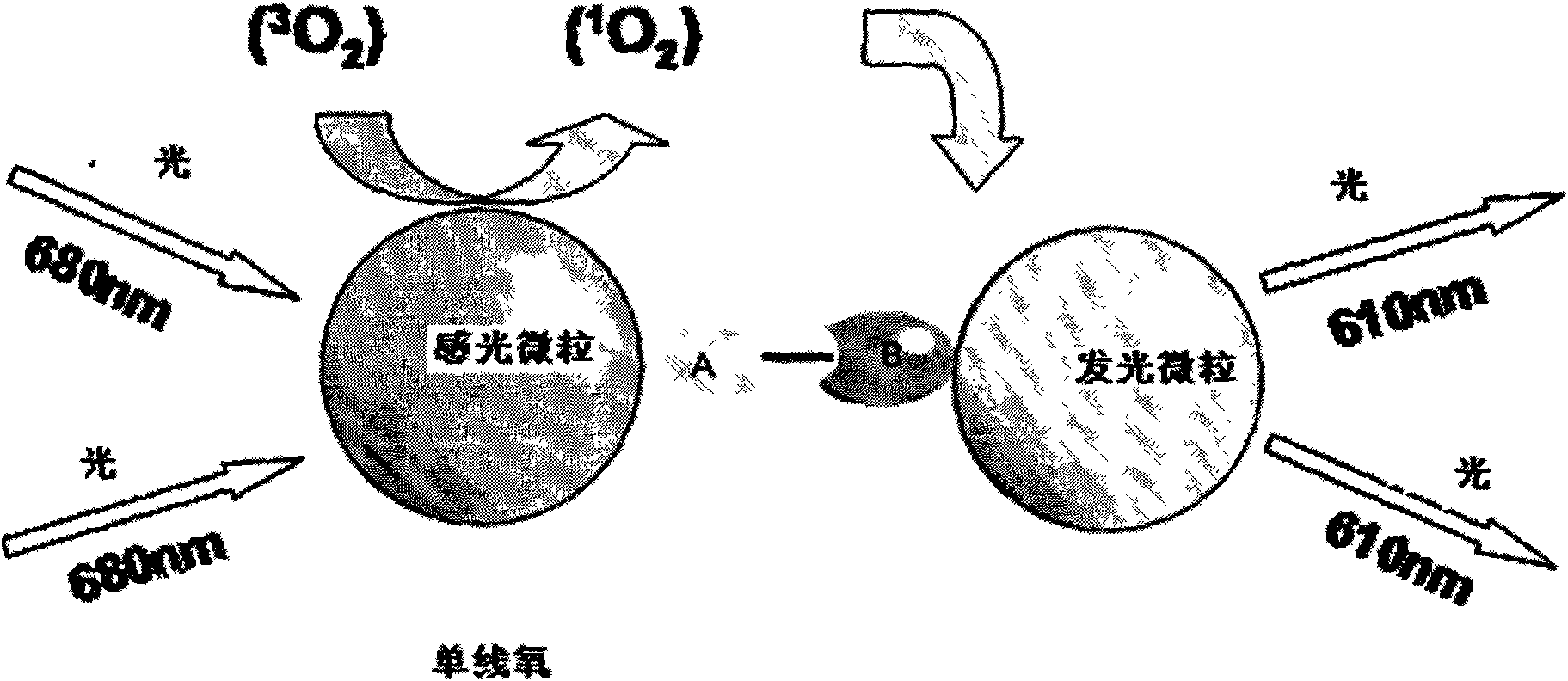
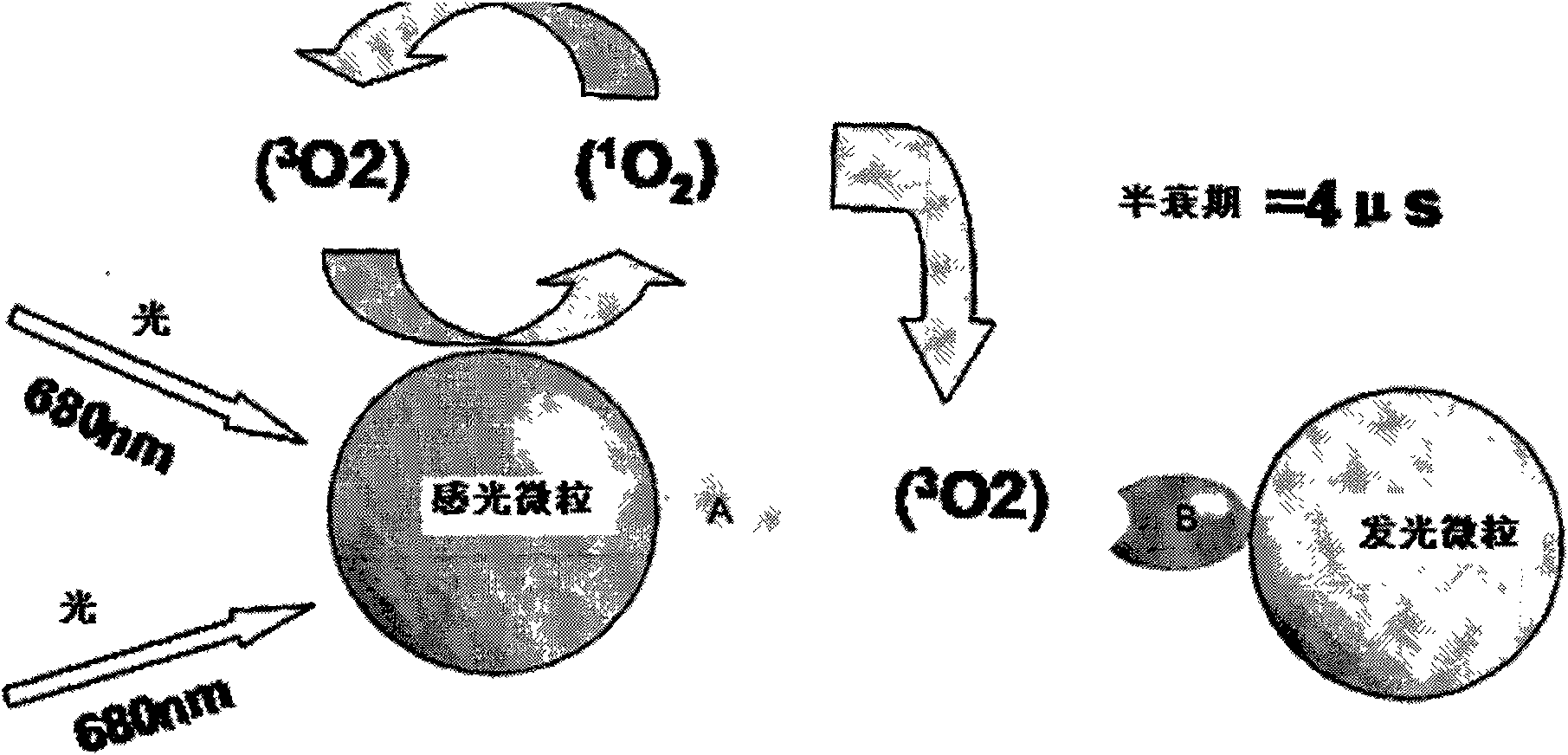
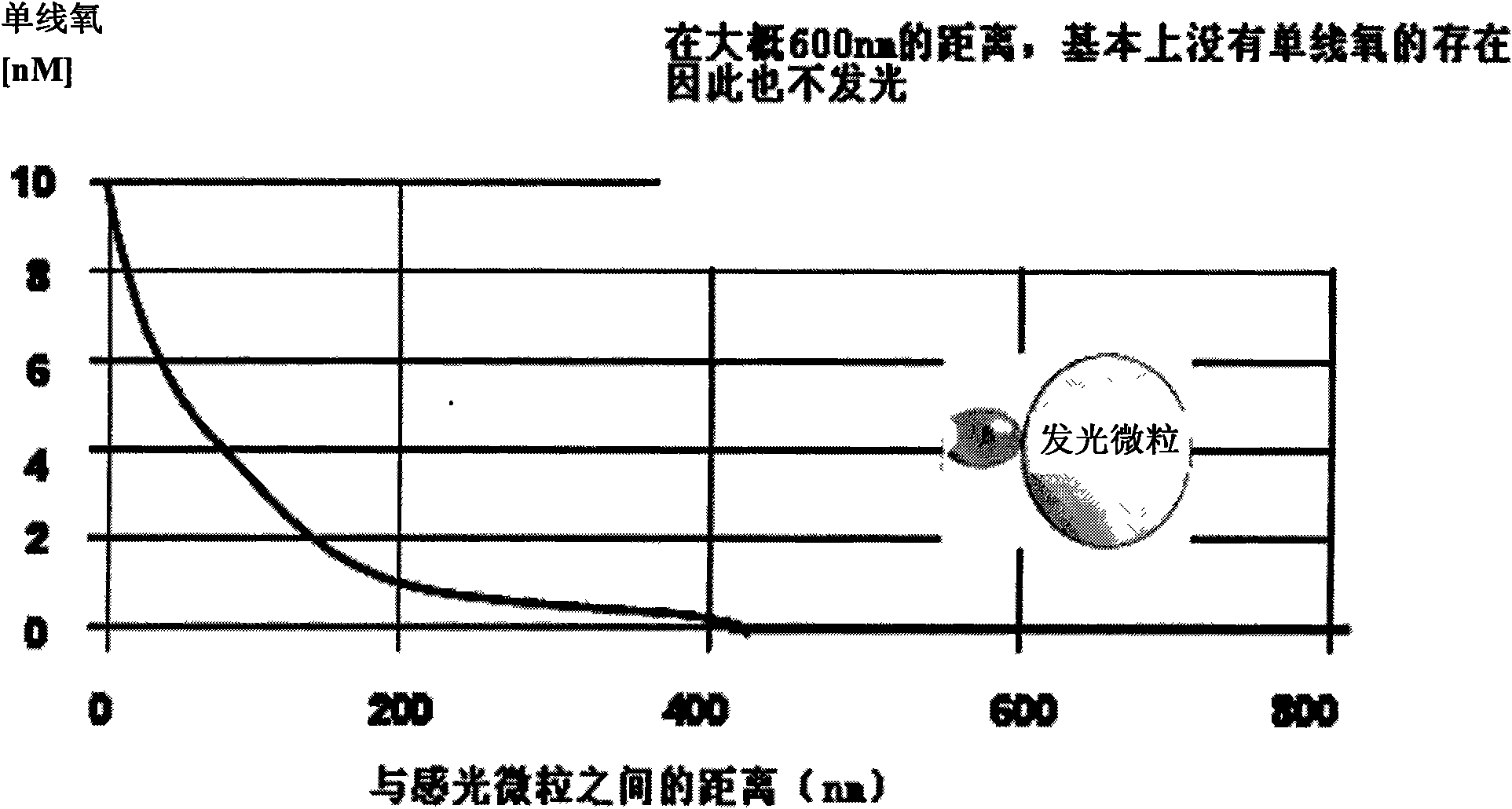
Hepatits B virus e antibody assay kit and assay method thereof
A hepatitis B and antibody detection technology, applied in the direction of biological testing, measuring devices, material inspection products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 Preparation of luminescent particles coated with anti-HBe antibody
[0085] Preparation:
[0086] 1) Treatment of luminescent particle suspension: absorb a certain amount of luminescent particles (particle size 250±20nm) and centrifuge in a high-speed refrigerated centrifuge, discard the supernatant, add a certain amount of MES buffer, ultrasonically break until the particles are resuspended, add MES buffer adjusted the concentration of luminescent microparticles to 100mg / ml.
[0087] 2) Antibody treatment: Anti-HBe was dialyzed in 0.05M MES buffer solution with pH 6.0 (hereinafter referred to as MES buffer solution). After the dialysis was completed, the concentration was measured and adjusted to 8 mg / ml.
[0088] 3) MES buffer, 100mg / ml luminescent particle suspension (MES buffer) and 8mg / ml anti-HBe (MES buffer) and mixed at a volume ratio of 1:2:5, and mixed quickly , to obtain the reaction solution.
[0089] 4) Prepare 25mg / ml NaBH with MES buffer 3 C...
Embodiment 2
[0127] Example 2 Preparation of biotin-labeled antibody
[0128] Preparation:
[0129] 1) Antibody treatment: dialyze anti-HBe in 0.1M NaHCO 3solution, the antibody concentration was measured and adjusted to 1 mg / ml.
[0130] 2) Prepare 16.17mg / ml Biotin solution with DMSO.
[0131] 3) Labeling: take the processed 1 mg / ml anti-HBe labeled antibody and the prepared Biotin solution, mix them according to the volume ratio of 10000:54, and mix them uniformly quickly. Stand at 2-8°C for 12-16 hours.
[0132] 4) Dialysis: Dialyze the reacted biotin-labeled antibody against biotin-labeled dialysis buffer (pH 8.00).
[0133] 5) Aspirate the dialyzed biotinylated antibody and transfer it to a clean centrifuge tube, and take a sample to determine the antibody concentration. Adjust the concentration of qualified biotin-labeled antibody to 0.5mg / ml.
[0134] According to the above preparation method, change the molecular ratio of antibody and Biotin to react and detect:
[0135] Op...
Embodiment 3
[0139] Example 3 Preparation of Photosensitive Microparticles Coated with Avidin
[0140] Photosensitive particles: use photosensitive particles with a particle size of 220±40nm (PentaTek, USA)
[0141] Preparation:
[0142] a. Treatment of photosensitive particle suspension: absorb a certain amount of photosensitive particles and centrifuge in a high-speed refrigerated centrifuge, discard the supernatant, add a certain amount of MES buffer, ultrasonically sonicate on the ultrasonic cell disruptor until the particles are resuspended, and then add MES buffer Adjust the photosensitive particle concentration to 100mg / ml.
[0143] b. Preparation of avidin solution: weigh a certain amount of Avidin, add MES buffer to dissolve to 8mg / ml.
[0144] c. Mixing: Mix the treated photosensitive microparticle suspension, 8 mg / ml Avidin and MES buffer at a volume ratio of 2:5:1, and mix quickly to obtain a reaction solution.
[0145] d. Reaction: Prepare 25mg / ml NaBH in MES buffer 3 CN s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com