Human immunodeficiency virus antigen and antibody determination kit and preparation method
A technology for human immunodeficiency and virus antigens, which is applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of unstable active area, inaccurate detection results, and increased detection time, so as to shorten the reaction time and reduce the loss of antigen activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] Preferred embodiments of the present invention are described below, and it should be understood that the preferred embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
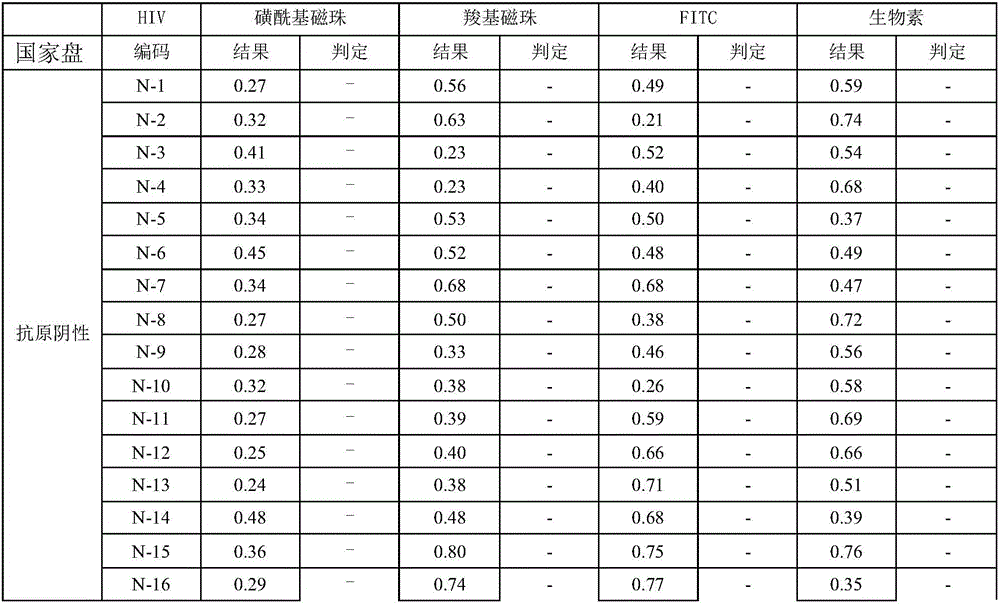
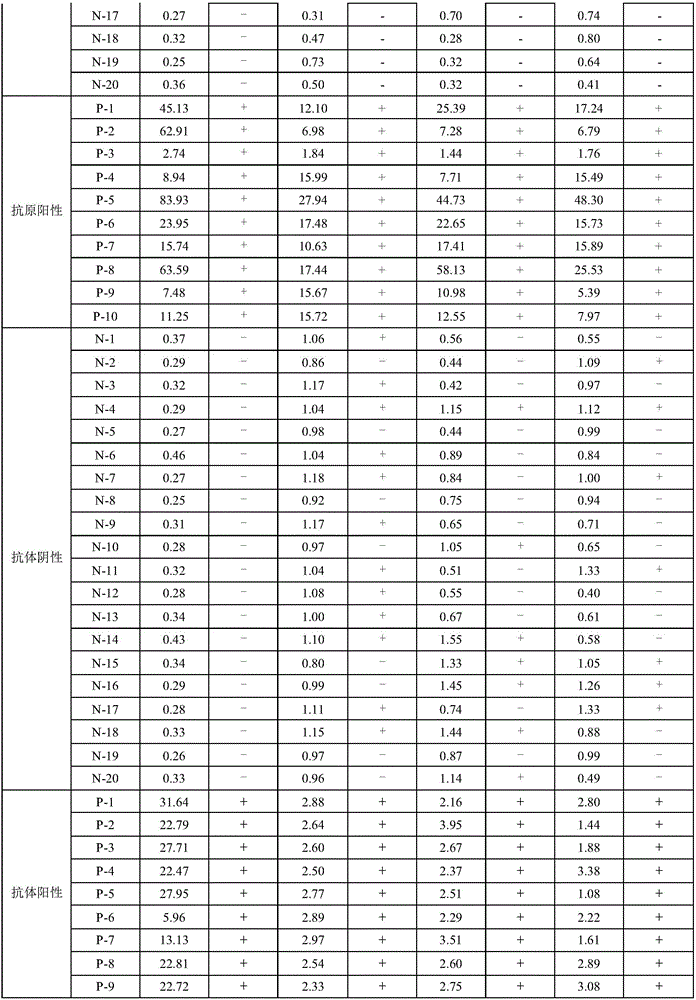
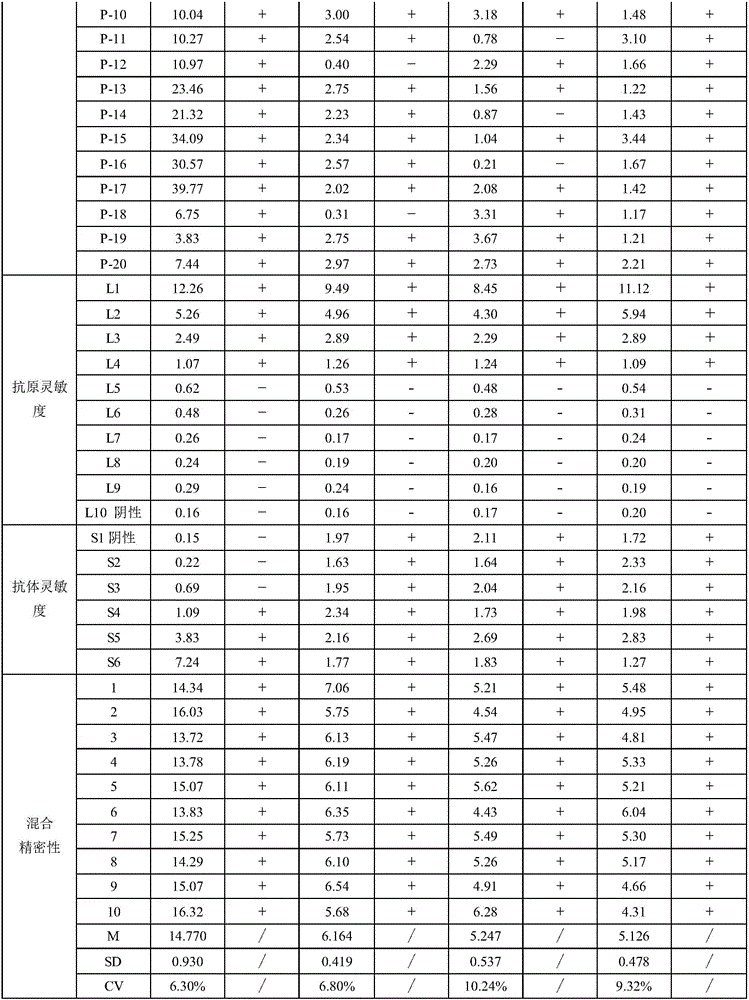
[0031] The present invention is a human immunodeficiency virus antigen and antibody assay kit for magnetic particle chemiluminescence method, which includes magnetic separation reagent, enzyme label reagent, negative / positive reference substance and calibration solution; the magnetic separation reagent is a combination of magnetic particle Coating antigen / antibody; the enzyme-labeled reagent is a detection antigen / antibody coupled alkaline phosphatase; wherein the coating antigen and detection antigen are recombinant antigens, and the coating antibody and detection antibody are mouse monoclonal antibodies; The standard solution contains the antigen to be tested and the antibody; the surface of the magnetic particle ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com