Kit for combined detection of hepatitis C virus antigen and antibody through chemiluminescence
A chemiluminescence and combined detection technology, applied in measurement devices, scientific instruments, instruments, etc., can solve the problems of missed diagnosis, obtaining test results, and complicated method operation, and achieve the effect of avoiding missed detection, ensuring specificity, and ensuring sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The components and concentrations of the detection reagents were
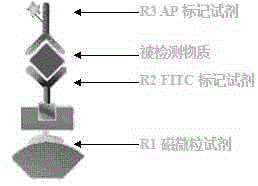
[0076] Component 1 (R1) magnetic particle reagent:
[0077] Magnetic particle and goat anti-FITC antibody conjugate 1%
[0078] Tris 12.1g / L
[0079] BSA 0.5g / L
[0080] PC-300 0.1%
[0081] Component 2 (R2) FITC labeling reagent:
[0082] FITC-labeled HCV-cAg antibody 1%
[0083] FITC-labeled NS3 protein 1%
[0084] FITC-labeled NS4 protein 1%
[0085] FITC-labeled NS5 protein 1%
[0086] Tris 12.1g / L
[0087] BSA 0.5g / L
[0088] PC-300 0.1%
[0089] Component 3 (R3) AP Labeling Reagent:
[0090] AP labeled HCV-cAg antibody 1%
[0091] AP-tagged NS3 protein 1%
[0092] AP-tagged NS4 protein 1%
[0093] AP-tagged NS5 protein 1%
[0094] Tris 12.1g / L
[0095] BSA 0.5g / L
[0096] PC-300 0.1%
[0097] Component 4 (R4) sample diluent:
[0098] Disodium hydrogen phosphate 1.974g / L
[0099] Potassium dihydrogen phosphate 0.2245g / L
[0100] BSA 0.5g / L
[0101] PC-300 0.1%
[0102] ...
Embodiment 2
[0135] The routine hepatitis C virus core antigen detection kit (enzyme-linked immunoassay) is selected from the American company Ortho, and the detection operation steps are as follows:
[0136] (1) Equilibration: Take out the kit and samples from the refrigerated environment, and place them at 15-30°C for 30 minutes;
[0137] (2) Dosing: Shake all reagents well before use, pay attention to no residual crystals in the bottle, and dilute the concentrated washing solution 20 times with distilled or deionized water;
[0138] (3) Adding samples: 2 wells each for blank, negative and positive controls are required for each test. Add 200 μl sample diluent to the blank well, first add 100 μl sample diluent to the other wells, then add 100 μl negative control to each well of the negative control well, add 100 μl positive control to each well of the positive control well, and add 100 μl sample to be tested to each well;
[0139] (4) Incubation: After mixing evenly, stick on the sealing ...
Embodiment 3
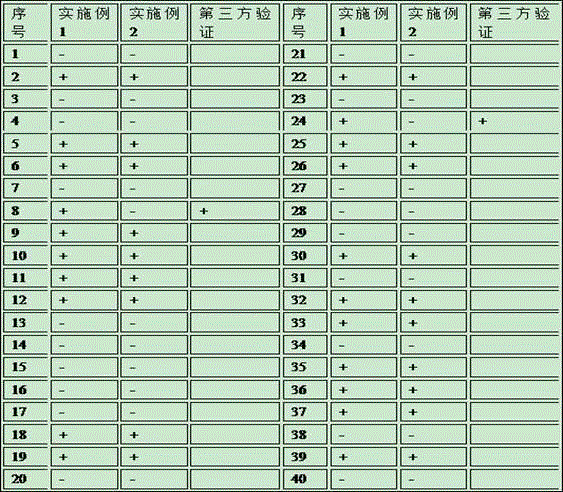
[0146] The accuracy of the kit for combined detection of hepatitis C virus antigen-antibody by chemiluminescence method was analyzed, and the kit of Ortho Company in the United States was used as the gold standard to detect 40 cases of clinical samples, and the results were compared. The detection method is as in Example 1 , embodiment 2, if there are differences in the test results, send a third-party inspection agency to verify by using the PCR detection method, and the test results are as shown in Table 1:
[0147] Table 1 Accuracy test results
[0148]
[0149] After comparative testing, the test results of No. 8 and No. 24 samples were abnormal. After the third-party test verification, the test results of Example 1 (the present invention) were more accurate. The two abnormal samples were mainly due to autoimmune antibodies, while The concentration of hepatitis C virus core antigen is relatively low, resulting in missed detection. According to the detection result, it ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com