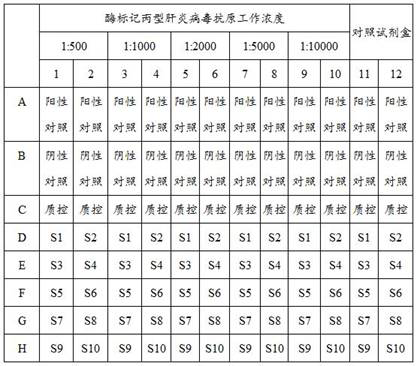
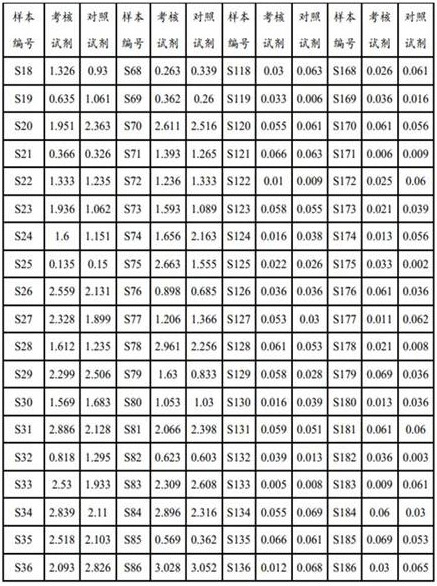
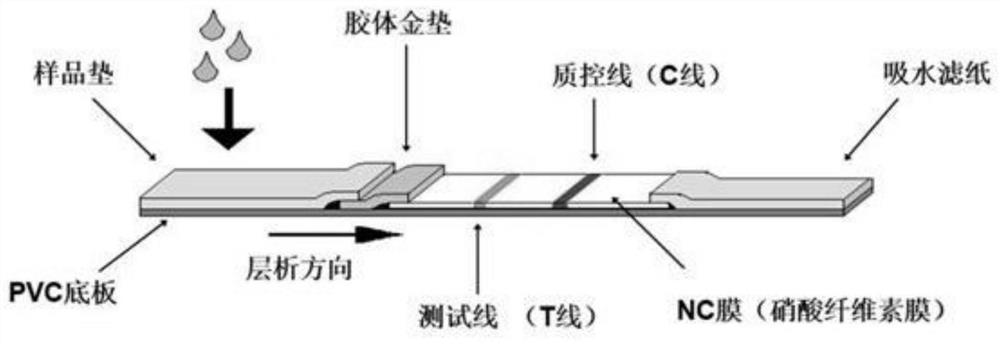
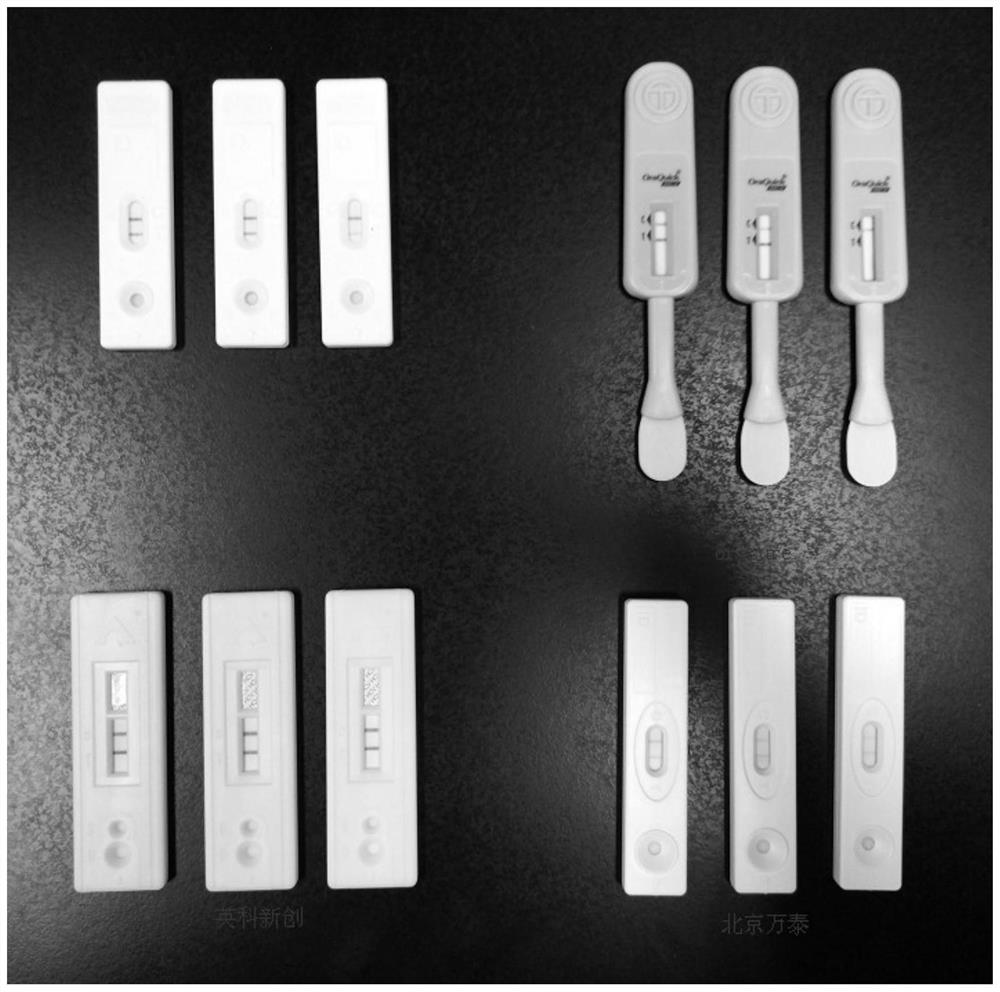
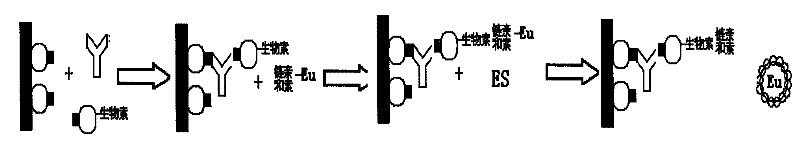Patents
Literature
41 results about "Hepatitis C virus Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hepatitis C Virus HCV Antibody. This test measures the level of hepatitis C antibodies. When the body is exposed to certain viruses, it produces antibodies in response to the infection. A high level of hepatitis C antibodies is considered a positive result and typically indicates either existing or previous infection with hepatitis C.1.
Hepatitis C virus antibody quick diagnosis test paper and its preparation method
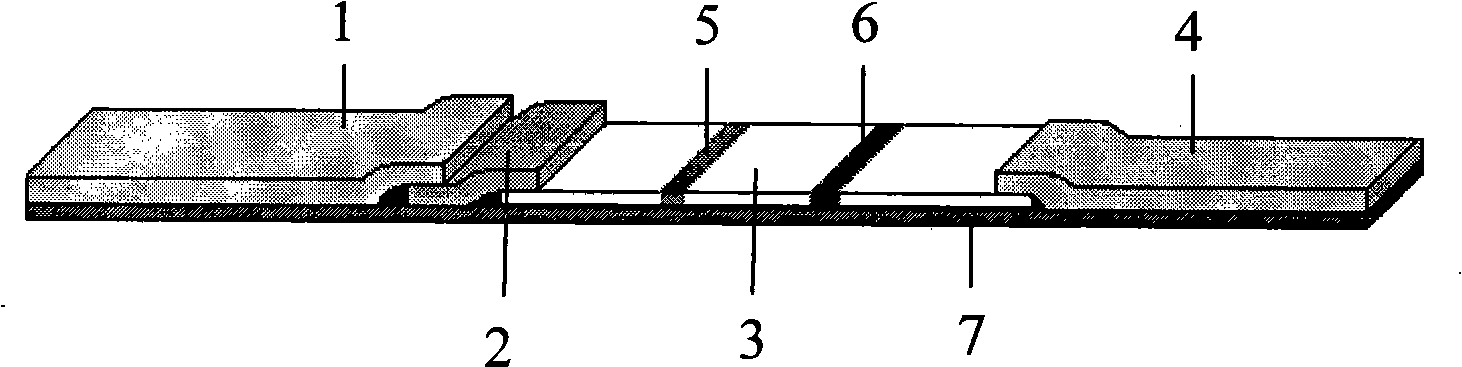
This invention discloses a kind of immunity chromatography test paper and its preparation method which can diagnose the type-C hepatitis virus fast, the said test paper includes the sampling tray, the gold size tray has labeled HCV mix antigen which links one end of the sampling tray closely, the pyroxylin film which links the other end of the gold size tray closely, and the suction sampling tray which links the other end of the pyroxylin film closely, the film encrusts the test (T) line and the quality control (C) line which are separate with each other, the sampling tray, the gold size tray, the film and the suction sampling tray are affixed in the plastic shoe plate to form the test paper, the said T line is the HCV mix antigen which is entrusted in the NC film, the said gold size tray has HCV recombination mix antigen, the said C line is the antibody of the HCV mix antigen entrusted in the film, it is used to test or clinical diagnosis of the HVC antigen, has the merits that the sensitive is high, the specificity is good, the operation is simple, the reaction is fast, it is fit to test in local and it is economical and useful.
Owner:天津中新科炬生物制药股份有限公司
Hepatitis C virus antibody diagnostic kit and preparation method thereof
ActiveCN102072957AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceSorbentQuality control
The invention belongs to the technical field of immunodiagnosis, in particular relates to a hepatitis C virus (HCV) antibody diagnostic kit adopting a micro particle chemiluminescence method, and a preparation method thereof. The kit consists of anti-HCV detecting magnetic micro particles, an anti-HCV detecting tracer conjugate, anti-HCV sample diluted solution and a quality control material. Theinvention also discloses the preparation method of the diagnostic kit, which adopts micro particle chemiluminescence immunoassay technology, has higher sensitivity and specificity than enzyme-linked immuno sorbent assay (ELISA), is suitable for clinical HCV auxiliary diagnosis and blood donor screening, and makes up the blank of the domestic production of the diagnostic kits for detecting the anti-HCV by the micro particle chemiluminescence method.
Owner:威海威高生物科技有限公司
Kit for detecting hepatitis c virus antibody as well as detection method and application thereof
ActiveCN104697988AHigh sensitivityStrong specificityChemiluminescene/bioluminescenceAntigenMicrosphere
The invention discloses a kit for detecting a hepatitis c virus antibody as well as a detection method and an application thereof, and belongs to the technical field of in vitro diagnosis and detection. The kit consists of the following components: (1) a magnetic microsphere system: including a magnetic microsphere and an HCV antigen in indirect connection by virtue of a first bridged compound; and (2) a marker system: including an HCV fusion antigen and a marking tracer which are in indirect connection by virtue of a second bridged compound, wherein the HCV antigen and the HCV fusion antigen are combined with an HCV antibody on different sites. The kit and the detection method, by detecting the hepatitis c virus antibody through chemiluminescence immunoassay, have the advantages of high sensitivity, good specificity and broad detection scope.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
Hepatitis virus type C immune body chemiluminescence method diagnostic reagent kit and its producing method
ActiveCN101196518AWide applicabilityLow costChemiluminescene/bioluminescenceBiological testingAntigenChemiluminescence
The invention relates to a diagnostic reagent kit for testing the hepatitis c virus (HCV) and the preparation and test method, which is to add the HCV recombinant antigen used for peridium into the buffer solution, blend it, move into the luminous microplate, make incubation for 18 hours under 4DEG.C, wash the luminous microplate, add into the confining liquid, leave the liquid after incubation and fully dry the luminous microplate to complete the preparation of the pre-peridium luminous microplate; combine the anti-human IgG used for marking and the horse radish peroxidase by improving the sodium periodate to complete the preparation of the enzyme marker; prepare the chemical luminous substrate solution A with luminal, Tween20 and luminous intensifier and prepare the chemical luminous substrate solution B with the hydrogen peroxide. The reagent kit also comprises the sample diluent and concentrated scrub solution. The negative corresponds to the normal human serum while the positive corresponds to the people with serum of pooled serum with HCV antibody. The reagent kit provided in the invention has much higher detection sensitivity than the ELISA, which is safe and reliable, easy to operate with low cost, and without any expensive full-automatic chemical luminous measuring apparatus required.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Reagent box for detecting nature of third type hepatitis virus antibody by using chemiluminescence method
InactiveCN101551397AHigh sensitivitySuit one's needsChemiluminescene/bioluminescenceBiological testingSubstance amountAntigen
The present invention discloses a reagent box for detecting nature of third type hepatitis virus antibody by using chemiluminescence method which includes an immunoreaction titration micropore plate and a detecting reagent, the immunoreaction titration micropore plate is a non-transparent polystyrol micropore plate; the detecting reagent includes third type hepatitis viruse antigen, enzyme label antihuman IgG, irradiancy substrates A liquor and irradiancy substrates B liquor. The reagent box uses chemiluminescence immune analysis method for detecting third type hepatitis virus antibody, the irradiancy substrate reacts chemically and releases mass energy by using enzyme catalysis irradiancy substrates for generating an excitation state intermediate body. When the excitation state intermediate body returns to a stable basic state, the excitation state intermediate body can transmit light quantum, utilize an irradiancy signal measurement instrumentation for measuring light quantum yield, the light quantum yield has a direct proportion with a waited-measured substance amount in sample. The method has high sensitivity, high precision and wide linear range; the reagent box has low manufacturing cost which can reduce patient burden and satisfy clinical requirement.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Specific binding substances for antibodies and their use for immunoassays or vaccines
The invention relates to derivatives of hepatitis C virus amino acid sequences. These derivatives can be used to screen samples, such as blood, to determine if antibodies to hepatitis C virus are present.
Owner:ROCHE DIAGNOSTICS GMBH
HCV IgM antibody rapid detection test paper
InactiveCN101266248ASimple and fast operationRapid responseMaterial analysisCelluloseAnti hcv antibody
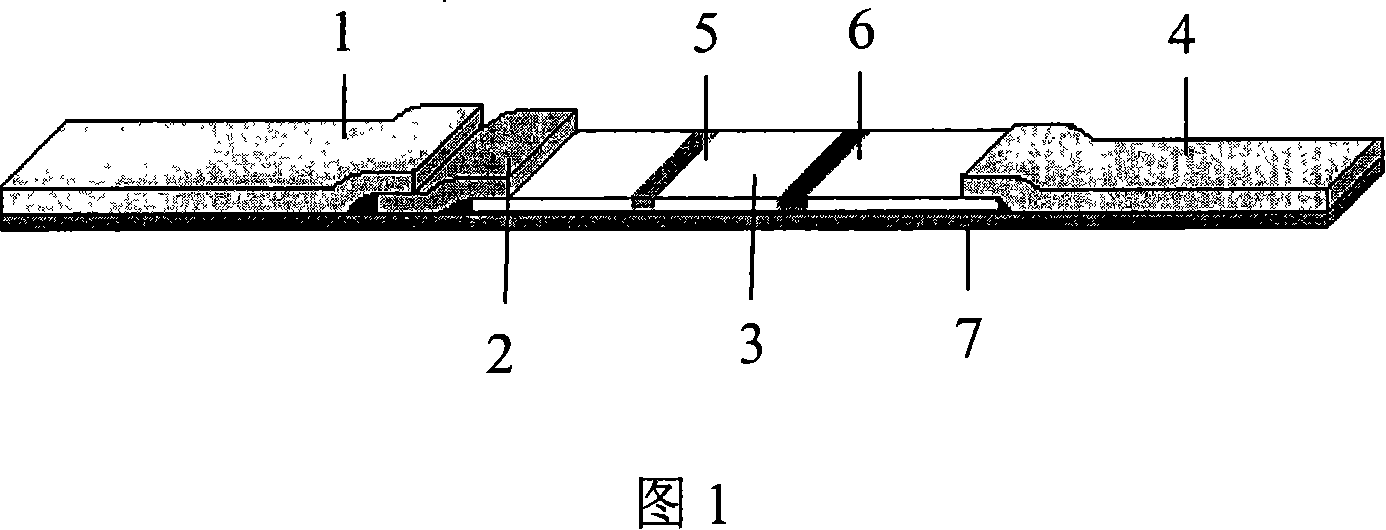
The invention relates to a biology applied technique field, especially relates to a hepatitis C virus antibody quick detection test paper, comprising a sampling pad (1); a colloidal gold pad (2) closely connected with one end of the sampling pad and containing HCV labelled mixed antigen; a cellulose nitrate (NC) membrane closely connected with another end of the sampling pad and a sucking sample pad (4) closely connected with another end of the NC membrane; a test T line (5) and quality control C line (6) mutually separated with each other and coated by NC membrane, wherein the T line is anti human IgM antibody coated on the NC membrane and the C line is anti HCV antibody coated on the NC membrane. During detecting, the detected sample is added on the sampling pad of the test paper and the immunoreaction result is directly observed to accomplish detection. The invention has features: high sensitivity, specificity, quickspeed, convenience and is suitable for detection on site and real time recording and storing the result.
Owner:天津中新科炬生物制药股份有限公司
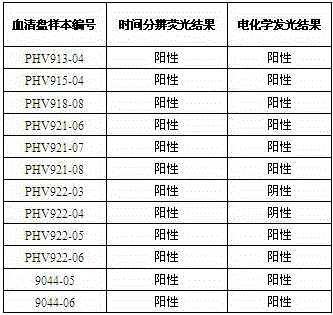
Hepatitis c virus antibody time-resolved fluoroimmunoassay and kit
InactiveCN102236020AHigh analytical sensitivityShort detection timeMaterial analysisAntigenPositive control
Owner:PERKINELMER MEDICAL DIAGNOSTICS PROD SHANGHAI
Kit for combined detection of hepatitis C virus antigen and antibody through chemiluminescence
InactiveCN104914244AAvoid missing detectionGuaranteed specificityBiological material analysisEnzyme immunoassaysImmunoresponse
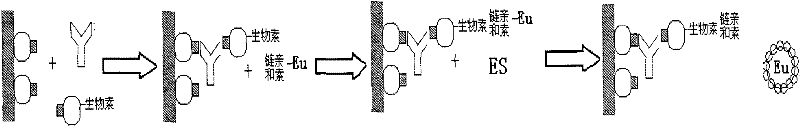
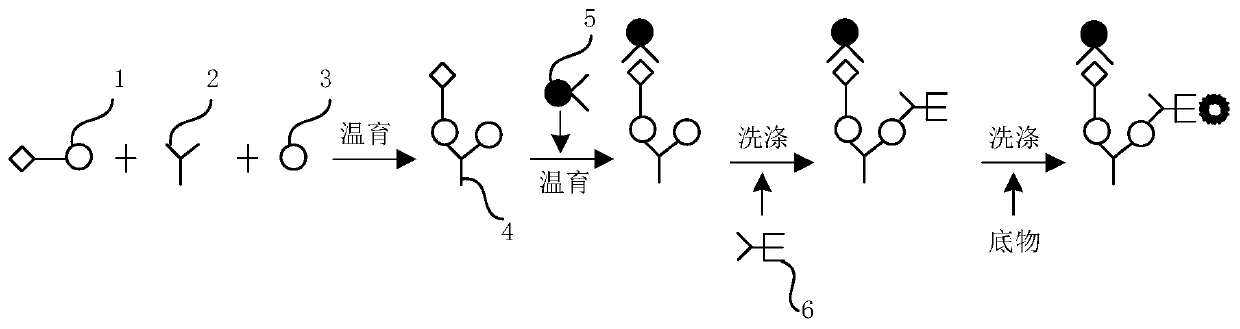
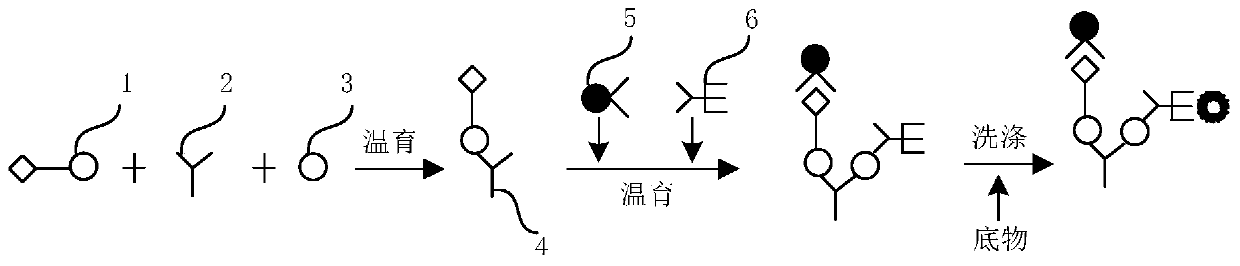
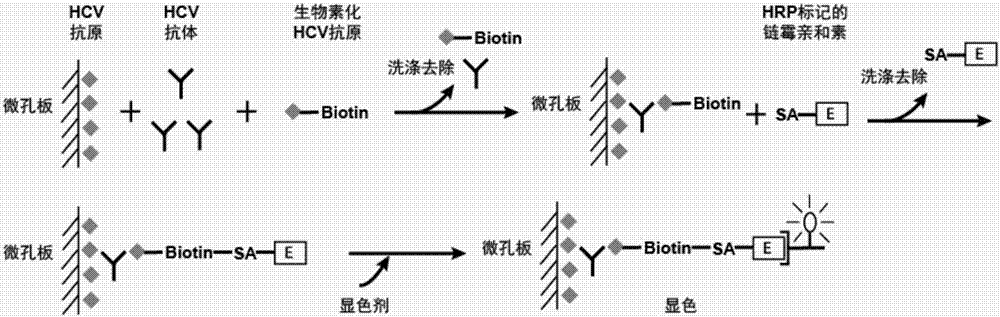
The invention provides a kit for combined detection of hepatitis C virus antigen and antibody through chemiluminescence. According to the invention, the principles of chemiluminescence are utilized; anti-FITC-coated magnetic particles are bonded with an FITC-coated reagent and then undergo immunoreaction with a detected object; after immunoreaction of an AP-labeled substance, an immunoreaction chain (as shown in a figure 1 which is described in the specification) is constructed; luminous sensitivity of a substrate catalyzed by AP is far higher than enzyme immunoassay development, so reaction sensitivity is enhanced; meanwhile, FITC is used to simultaneously marking antigen and antibody, so hepatitis C virus antibody and core antigen are detected at the same time. The kit provided by the invention can more accurately detect hepatitis C, is free of leak detection, achieves the effect of early discovery and has a high application value in prevention of hepatitis C.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Method for detecting hepatitis C virus antibody
InactiveCN1755365AStrong specificityIncreased sensitivityMaterial analysis by observing effect on chemical indicatorAntigenPeroxiredoxin
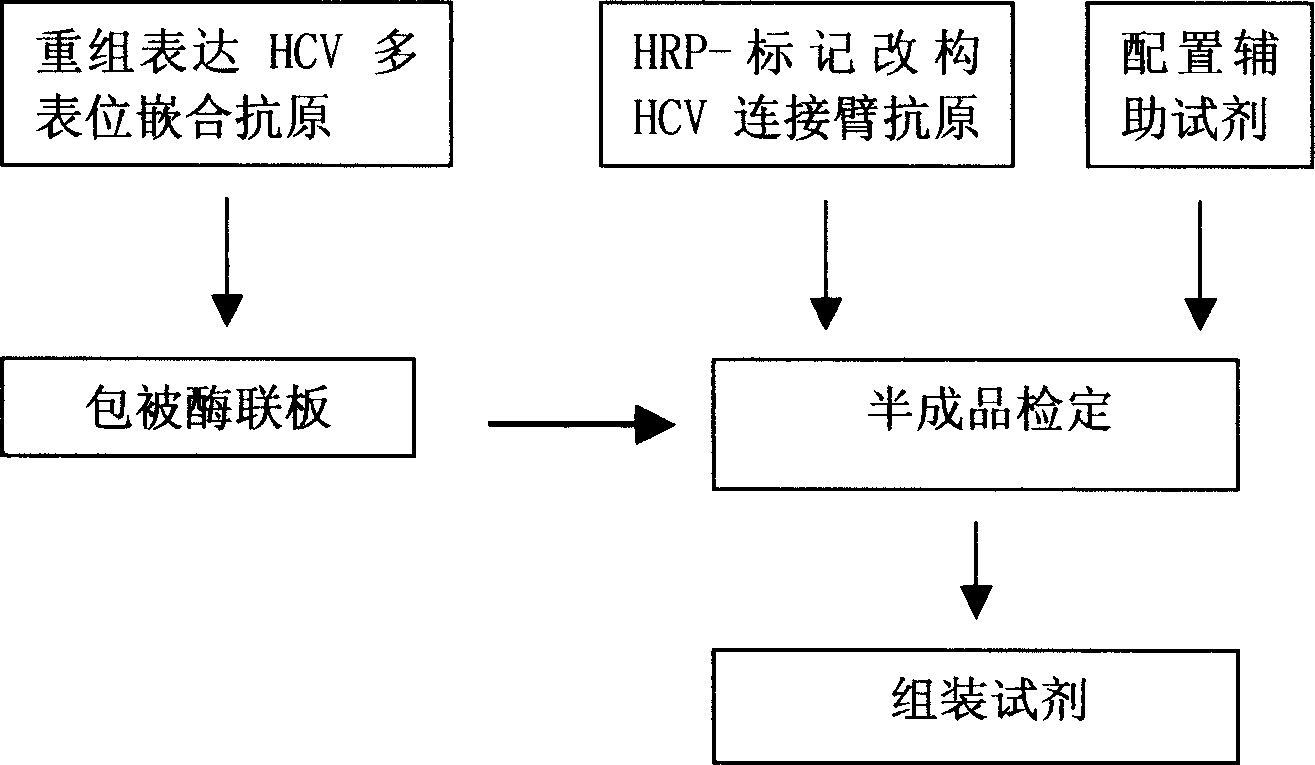
The invention discloses a method for adopting enzyme immune technology to test hepatitis C virus antibody, it also discloses the hepatitis C virus multiple bit table jogging antibody and the enzyme label connecting arm. The testing method comprises: preparing the hepatitis C virus multiple bit table jogging antibody, preparing the jogging antibody and the label connecting arm, preparing the jogging antibody label horse radish peroxides compound, using the jogging antibody to uterus the enzyme checker board, adding tested antibody and enzyme label antibody compound and so on.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Reagent for high throughput combined detection of hepatitis c virus antigen-antibody
ActiveCN105004862AIntuitive identification of infection periodHigh sensitivityBiological testingFluorescence/phosphorescenceAntigenFluorescein
The invention provides a reagent for high throughput combined detection of hepatitis c virus antigen-antibody. By the flow cytometry theory, different concentrations of FITC fluorescein-labeled microspheres are correspondingly coated with antibody and antigen; after addition of a detection sample, the microspheres, the detection sample and the PE-labeled paired antibody and antigen form a sandwich structure; and under the action of 480nm exciting light, the effects of simultaneous detection of hepatitis c virus antigen-antibody can be achieved in a high throughput way according to different emitted light intensities of fluoresceins with different concentrations. By the utilization of fluorescent color-developing effect, reaction sensitivity is enhanced, and hepatitis c virus antibody and core antigen can be detected simultaneously by the utilization of FITC-labeled microspheres for simultaneous detection of antigen-antibody. The reagent is more accurate in hepatitis c detection, has no defect in detection leakage, achieves the early detection effect and has high application value in prevention of hepatitis c.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
Integral detection reaction plate and protein chip kit of hapetitis and cirrhosis
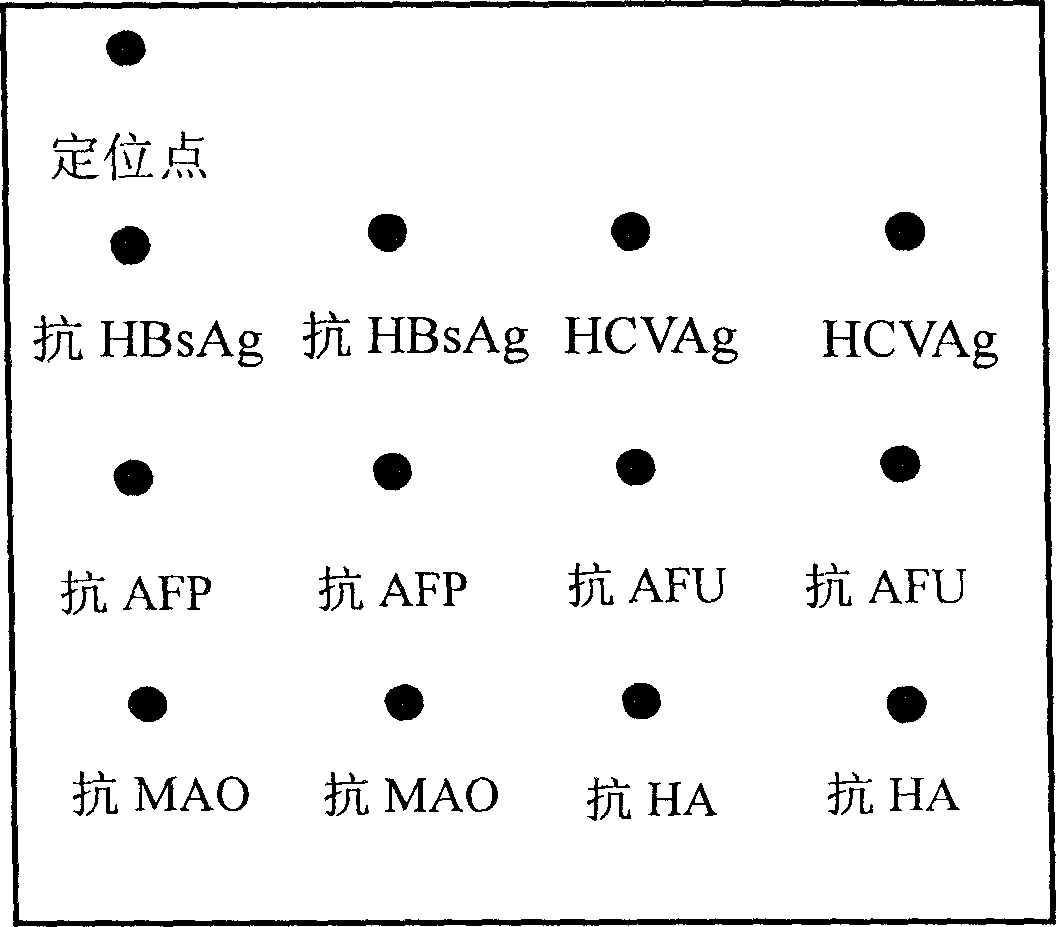
The invention relates to an integrated detection reaction orifice plate and protein chip reagent box for detecting six indexes of diagnosis, forecast and prognosis of hepatitis, hepatocirrhosis and liver cancer, including hepatitis B virus surface antigen (HBsAg), hepatitis C virus antibody (HCVAb), alpha-fetoprotein (AFP), alpha-L-Fucosidase (AFU), mono amine oxidase (MAO), hyaluronic acid (HA). And the orifice plate comprises a substrate and reaction orifices on the substrate, where the reaction orifices comprise 2-384 sample orifices and 2-8 standard product orifices, and at the bottom of each reaction orifice is solid carrier coated with micro lattice of HBsAg, HCVAg, AFP, AFU,MAO,and HA antigens / antibodies or more. And the reaction plate and reagent can simply and conveniently, quickly and accurately implement simultaneous detection of the six indexes of diagnosis, forecast and prognosis of hepatitis, hepatocirrhosis and liver cancer for many persons.
Owner:穆海东
Oral fluid rapid assay for hepatitis C virus (HCV) antibodies using non-antibody labeling of IgA molecules recognizing HCV peptide epitopes
InactiveUS20050009011A1Easy to detectHigh detection sensitivityMicrobiological testing/measurementBiological testingPeptide antigenAntigen binding
A method and device to detect Hepatitis C (HCV) antibodies in oral fluid is provided. This method introduces a non-antibody detection molecule that labels all classes of patient antibodies in oral fluid, followed by the specific concentration of labeled anti-HCV antibodies by selective capture in a trapping zone consisting of peptide antigens derived from the HCV genome. Signal generated by the labeled antibodies present in the trapping zone is proportional to the number of anti-HCV antibodies bound to the antigens present in the trapping zone. Presence of signal derived from the capture of antibody / detection molecule complexes in the trapping zone is indicative of past exposure to HCV.
Owner:INSTANT MEDICAL DIAGNOSTICS
Hepatitis c virus antibodies
ActiveUS20110002926A1Reduced activityHigh activityImmunoglobulins against virusesAntiviralsHepatitis C virus E2Humanized antibody
Owner:MEDICAL RESEARCH COUNCIL TECHNOLOGY
Method for detecting hepatitis C virus antibody in serum
InactiveCN102305862ARealize quantitative detectionIncreased sensitivityBiological testingAntigenReporter gene
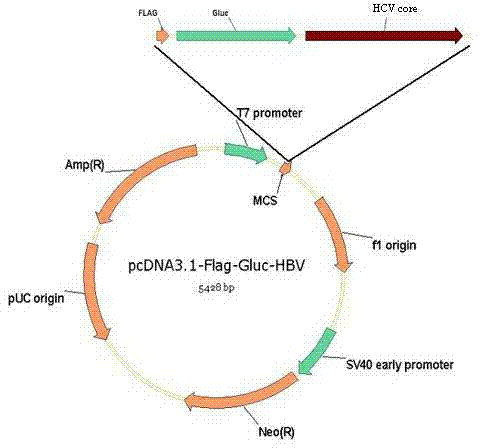
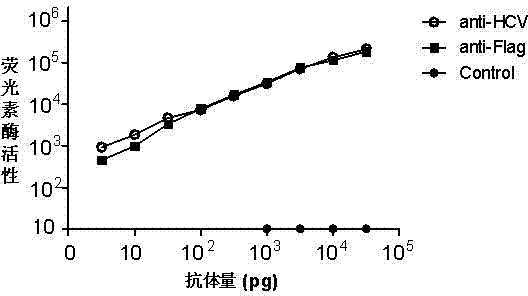
The invention belongs to the field of biotechnology and in particular relates to a method for detecting hepatitis C virus (HCV) antibody in serum, which comprises the following steps of: constructing an HCV gene and reporter gene recombinant plasmid provided with tagged protein gene, introducing the recombinant plasmid into a mammal cell line for expression, purifying fusion protein by using the tagged protein antibody, incubating the fusion protein and the serum, capturing an antigen-antibody complex by using immunomagnetic beads, and adding a reporter gene substrate to detect reporter gene activation level so as to reflect the content of the HCV antibody.
Owner:方辉
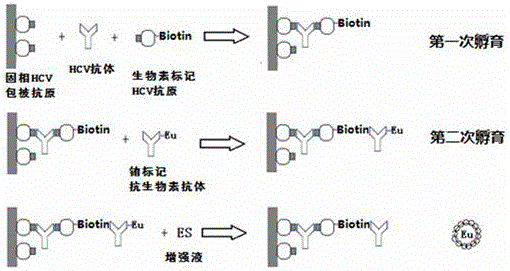
Antibody to hepatitis C virus time resolution detection kit and preparation method thereof
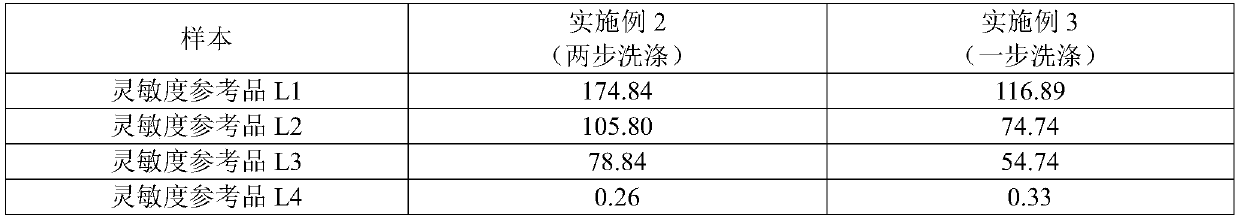
The invention discloses an antibody to hepatitis C virus time resolution detection kit, wherein s specific recombinant antigen is selected to coat and mark, thereby optimizing a sealing process and improving the specificity of the kit; after signs are systematically optimized by utilizing biotin species and sign process, the sensitivity of the kit is further improved; therefore, the sensitivity is high, the specificity is good, and meanwhile, compared with the imported luminescence reagent, the detection cost is largely reduced.
Owner:SUZHOU SYM BIO LIFESCI CO LTD
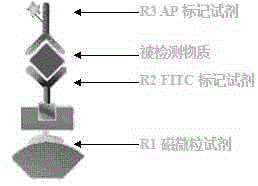
Hepatitis C virus antibody detection kit, preparation method and detection method
PendingCN111579781AFully combinedHigh detection sensitivityChemiluminescene/bioluminescenceAntibody combining siteBinding site
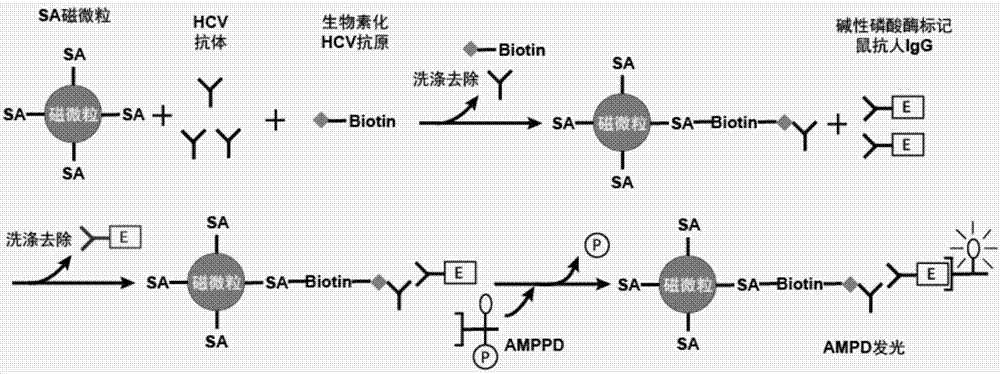
The invention relates to the technical field of in-vitro diagnosis and detection, in particular to a hepatitis C virus antibody kit, a preparation method and a detection method. The hepatitis C virusantibody kit comprises a reagent R1, a reagent R2 and a reagent R3, and the reagent R1 comprises a streptavidin magnetic particle solution; the R2 reagent comprises a mixed reagent of a biotinylated hepatitis C virus antigen and a hepatitis C virus recombinant antigen; and the R3 reagent comprises an alkaline phosphatase labeled hepatitis C virus monoclonal antibody solution. According to the invention, the hepatitis C virus antibody, the biotinylated hepatitis C virus antigen and the hepatitis C virus recombinant antigen form a 'sandwich' sandwich compound, so that the blocking of antigen andantibody binding sites due to the addition of magnetic particles and enzyme markers is avoided, and the sensitivity and specificity of the detection of the hepatitis C virus antibody are remarkably improved.
Owner:深圳市爱康试剂有限公司
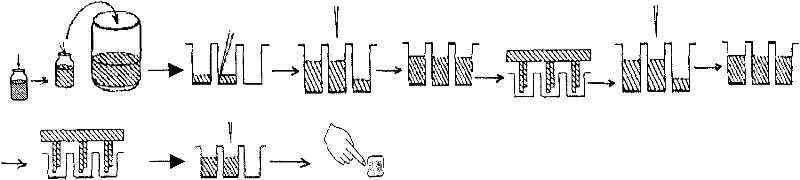
Preparation method of hepatitis c virus antibody detection test paper
The invention discloses a preparation method of a HCV (hepatitis c virus) antibody detection test paper. The preparation method comprises the following steps of using a mixed liquid labeled by HCV labeled monoclonal antibody and HCV labeled antigen to spray gold to a fiber cellulose membrane by membrane dotting and gold spraying equipment, so as to obtain a gold pad; using a HCV-coated antigen detection line coating liquid and a contrast line coating liquid to respectively score on a nitrate cellulose membrane, treating, and adhering with a polyvinyl chloride substrate, so as to obtain the membrane-dotted polyvinyl chloride substrate; assembling a sample pad, the gold pad, the membrane-dotted polyvinyl chloride substrate and water absorbing paper, and cutting into the detection test paper. By adopting the type, the prepared detection test paper has the advantages that on the basis of immune lateral flow chromatography technique, the containing of HCV antibody in a blood sample can be judged by manual operation and naked eye reading, the diagnosis speed is quick, the result is accurate, and the injury to a body of the detected person is avoided.
Owner:SUZHOU WANMUCHUN BIOLOGICAL TECH
Kit for detecting hepatitis C virus antibody, detection method and application thereof
ActiveCN104697988BHigh sensitivityStrong specificityChemiluminescene/bioluminescenceAntigenMicrosphere
The invention discloses a kit for detecting a hepatitis c virus antibody as well as a detection method and an application thereof, and belongs to the technical field of in vitro diagnosis and detection. The kit consists of the following components: (1) a magnetic microsphere system: including a magnetic microsphere and an HCV antigen in indirect connection by virtue of a first bridged compound; and (2) a marker system: including an HCV fusion antigen and a marking tracer which are in indirect connection by virtue of a second bridged compound, wherein the HCV antigen and the HCV fusion antigen are combined with an HCV antibody on different sites. The kit and the detection method, by detecting the hepatitis c virus antibody through chemiluminescence immunoassay, have the advantages of high sensitivity, good specificity and broad detection scope.
Owner:SHENZHEN NEW INDS BIOMEDICAL ENG
Hepatitis C antigen pretreatment method, and detection kit
ActiveCN107247144AIncreased ability to bind HCV antibodiesHigh sensitivityChemiluminescene/bioluminescenceAids diagnosticsBiotin
The invention discloses a hepatitis C antigen pretreatment method, a preparation method of a biotinylated hepatitis C antigen, and a hepatitis C virus antibody detection kit comprising the biotinylated hepatitis C antigen. The hepatitis C antigen is pretreated to change the protein conformation, and the pretreated hepatitis C antigen reacts with biotin to produce the biotinylated hepatitis C antigen. The hepatitis C virus antibody detection kit adopts the biotinylated hepatitis C antigen prepared through a reaction of the pretreated hepatitis C antigen and biotin, so the detection ability of the hepatitis C antibody is greatly improved, the specificity is improved, and the kit also has the advantages of good stability and easiness in production control, so the kit is massively suitable for the assisted diagnosis and blood screening of the hepatitis C virus.
Owner:SHANGHAI KEHUA BIO ENG
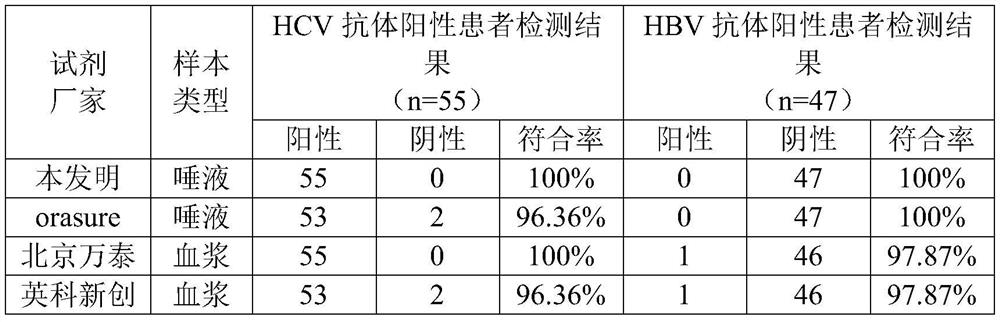
Hepatitis C virus antibody saliva detection kit
The invention discloses a hepatitis C virus antibody saliva detection kit. The kit includes a flowering gold labeled recombinant protein GL (rGL) and a quality control antigen on glass fiber, hepatitis C antigen detection, a recombinant HCV Ag and a quality control monoclonal antibody coating a detection line and a quality control line of nitrocellulose membrane, and a specific antibody for detecting anti-hepatitis C virus in human saliva. The kit provided by the invention has high total consistent rate, can be used for non-invasive detection, and is convenient for invasive detection of serum, at the same time the result display is clear, and the detection efficiency is higher.
Owner:河南爱微迪生物技术有限公司
Hepatitis C virus antibodies
ActiveUS9193781B2Reduced activityHigh activityImmunoglobulins against virusesAntiviralsHepatitis C virus E2Humanized antibody
Owner:MEDICAL RESEARCH COUNCIL TECHNOLOGY
Hepatitis C virus antigen-antibody joint detection method and kit
The invention relates to a hepatitis C virus antigen-antibody joint detection method in the technical field of immunodetection. The kit comprises at least two detection areas, whether a first compoundformed by a donor-hepatitis C virus antibody-receptor exists or not is detected in one detection area, and whether a second compound formed by a donor-hepatitis C virus antigen-receptor exists or notis detected in the other detection area, a donor can generate active oxygen in an excited state, and a receptor can react with the active oxygen to generate a detectable chemiluminescence signal. Themethod is advantaged in that the HCV core antigen and the antibody are jointly detected, so detection accuracy is improved, detection cost is low, moreover, a treating agent is added into a core antigen detection hole, so interference of a low-affinity antibody in an early body is reduced, the antigen detection sensitivity in a conversion period is improved, and the HCV detection sensitivity is further improved.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Hepatitis C virus antigen fluorescent immunochromatography detection kit and preparation method therefor
PendingCN109324184ARapid Quantitative DetectionConvenient Quantitative DetectionMaterial analysisDiluentCapture antibody
The invention discloses a hepatitis C virus antigen fluorescent immunochromatography detection kit and a preparation method therefor. The detection kit comprises the following components: a fluorescent microsphere labeled hepatitis C virus antibody 1, a hepatitis C virus antibody 2, a goat anti-mouse capture antibody, a phosphate buffer, and a sample diluent. The preparation method for the detection kit comprises the following steps: preparing the sample diluent; preparing the fluorescent microsphere labeled hepatitis C virus antibody 1; preparing a carrier 1 coated with the fluorescent microsphere labeled hepatitis C virus antibody 1; preparing a carrier 2 coated with the hepatitis C virus antibody 2 and the goat anti-mouse capture antibody; and assembling the standard substance to obtainthe fluorescent immunochromatography detection kit. The detection kit can rapidly and conveniently carry out quantitative detection on hepatitis C virus antigens on site, and has good detection sensitivity, precision and accuracy.
Owner:XIAMEN TONGRENXIN BIO-TECH CO LTD
Hepatitis C virus antigen-antibody joint inspection kit and application thereof
PendingCN111521780AReduced detection windowReduce distractionsMaterial analysisReceptorAntigen testing
The invention relates to a hepatitis C virus antigen-antibody joint detection kit and an application thereof, belongs to the technical field of immunodetection. The kit comprises the following components, a receptor bound with a first antigen, a second antigen, a receptor binding to the first antibody, and a second antibody, wherein the first antigen and the second antigen can be specifically combined with a variable region of a hepatitis C virus antibody, the first antibody and the second antibody can be specifically combined with different epitopes of a hepatitis C virus antigen. The kit isadvantaged in that the HCV antigen-antibody joint detection kit can be used for joint detection of HCV core antigen and antibody, so a detection window period is shortened, detection cost is low, moreover, the kit further comprises a treating agent, and the treating agent can reduce the interference of a low-affinity antibody in an early body and improve the antigen detection sensitivity in a conversion period, so the HCV detection sensitivity is improved, and the HCV detection window period is further shortened.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Hepatitis C virus antibody diagnostic kit and preparation method thereof
ActiveCN102072957BHigh sensitivityStrong specificityChemiluminescene/bioluminescenceSorbentTrue positive rate
The invention belongs to the technical field of immunodiagnosis, in particular relates to a hepatitis C virus (HCV) antibody diagnostic kit adopting a micro particle chemiluminescence method, and a preparation method thereof. The kit consists of anti-HCV detecting magnetic micro particles, an anti-HCV detecting tracer conjugate, anti-HCV sample diluted solution and a quality control material. Theinvention also discloses the preparation method of the diagnostic kit, which adopts micro particle chemiluminescence immunoassay technology, has higher sensitivity and specificity than enzyme-linked immuno sorbent assay (ELISA), is suitable for clinical HCV auxiliary diagnosis and blood donor screening, and makes up the blank of the domestic production of the diagnostic kits for detecting the anti-HCV by the micro particle chemiluminescence method.
Owner:威海威高生物科技有限公司
Hepatitis virus type C immune body chemiluminescence method diagnostic reagent kit and its producing method
ActiveCN101196518BHigh detection sensitivitySimple and fast operationChemiluminescene/bioluminescenceBiological testingAntigenHorse radish peroxidase
Owner:CHEMCLIN DIAGNOSTICS CO LTD
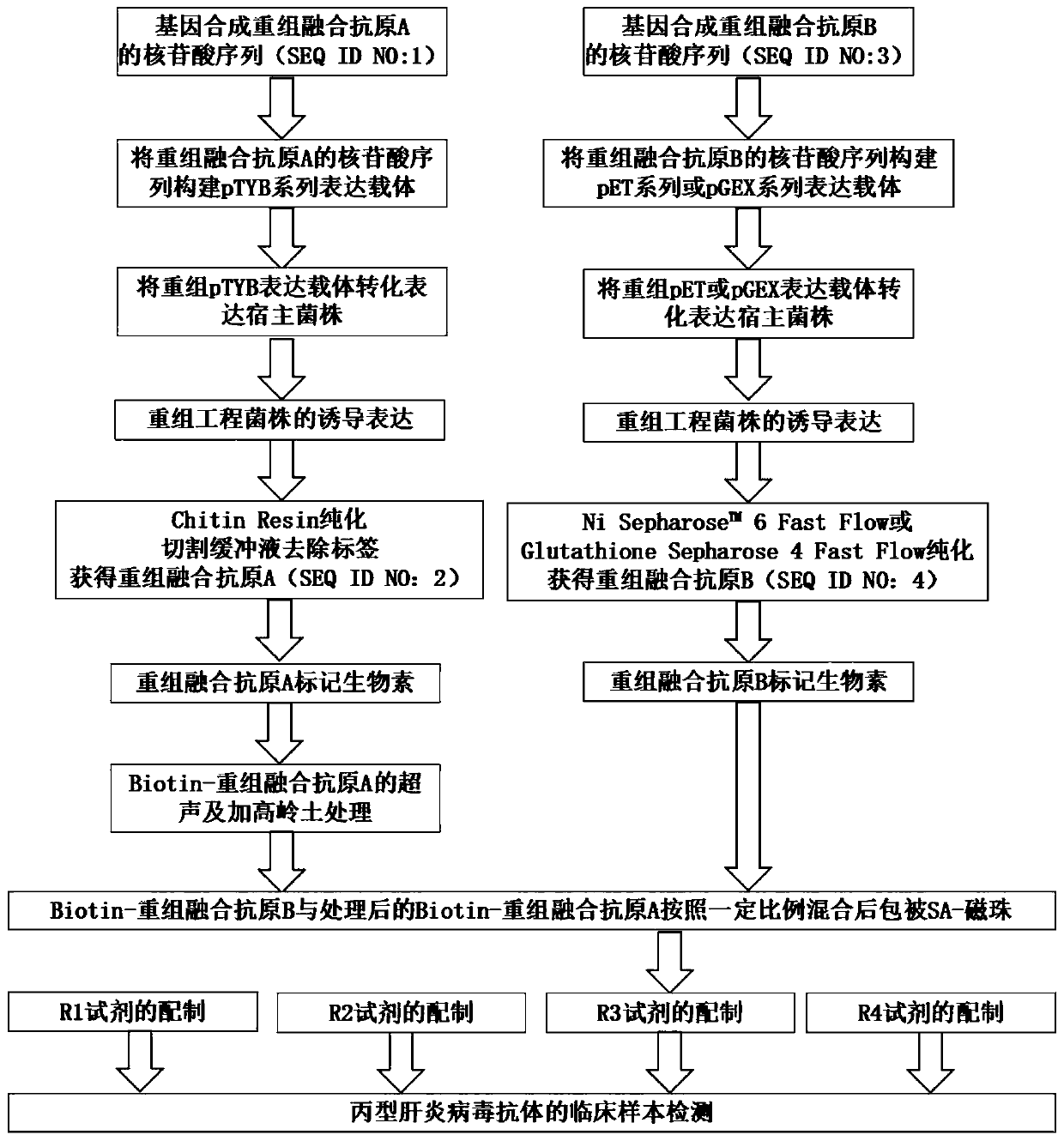
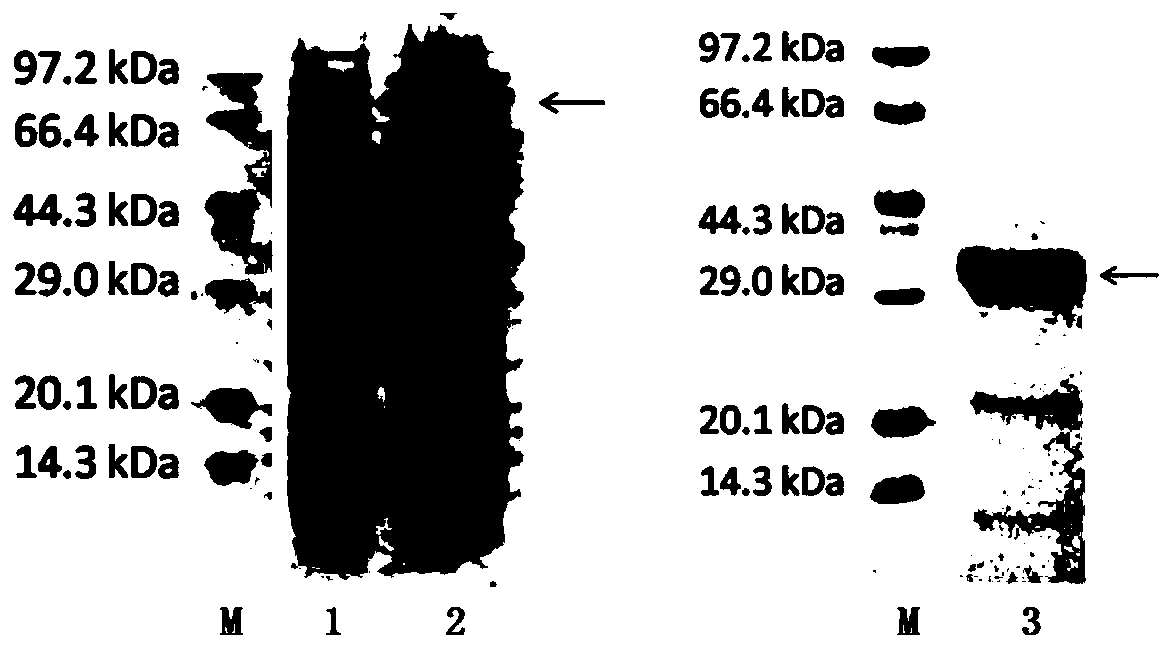
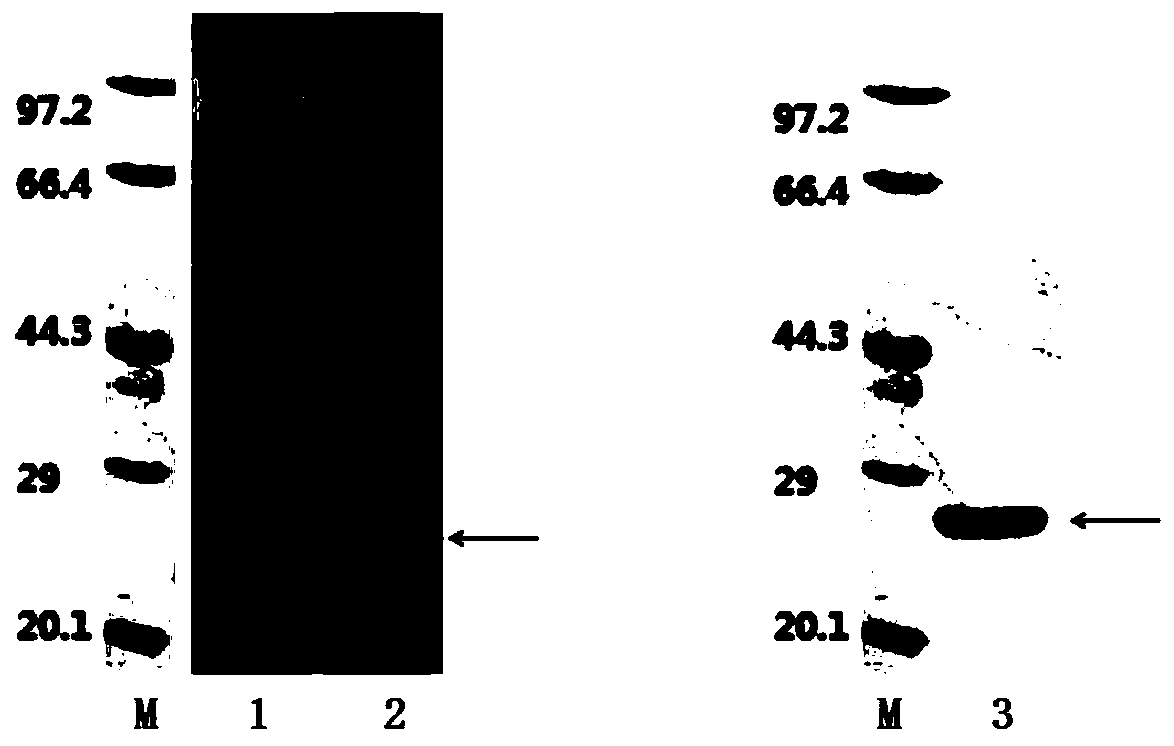
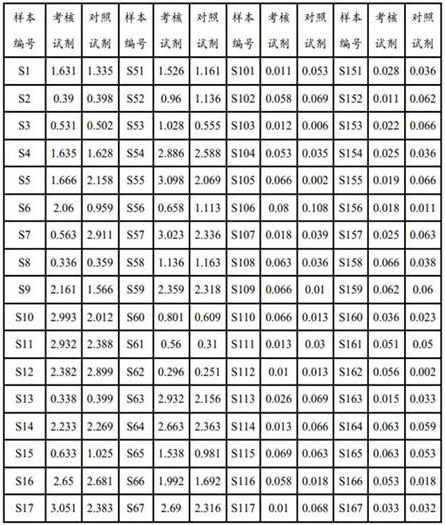
A hepatitis C virus antibody detection reagent comprising recombinant fusion antigen a and b and its application and recombinant fusion antigen a and b
ActiveCN108196069BImprove solubilityFold preciselySsRNA viruses positive-senseAntibody mimetics/scaffoldsChylomicronHcv hepatitis c virus
The invention relates to an HCV (hepatitis c virus) antibody detection reagent containing recombinant fusion antigens A and B, an application and the recombinant fusion antigens A and B, and belongs to the technical field of bioengineering. According to the HCV antibody detection reagent containing the recombinant fusion antigens A and B, the recombinant fusion antigen A contains an amino acid sequence as shown in SEQ ID NO: 2; the recombinant fusion antigen B contains an amino acid sequence as shown in SEQ ID NO: 4; the detection reagent comprises a reagent R1, a reagent R2, a reagent R3 anda reagent R4. The detection reagent has the following advantages: the two recombinant fusion antigens have the characteristics of high specificity and good stability and can be used for preparing thehepatitis C virus antibody detecting reagent by mixing in a certain ratio, and the clinical detection effect of the reagent is remarkable. Meanwhile, the detection reagent has high anti-interference capacity to chylomicron, bilirubin and hemoglobin, and can greatly meet requirements of clinical diagnosis of HCV.
Owner:深圳德睿生物科技有限公司
Hepatitis C virus antibody detection kit and application thereof
InactiveCN113189348AStrong specificityGood repeatabilityChemiluminescene/bioluminescenceBiological testingAntigenEnzyme binding
The invention discloses a hepatitis C virus antibody detection kit and application thereof. The hepatitis C virus antibody detection kit comprises a hepatitis C virus antigen coated plate, an enzyme conjugate and a chemiluminescent substrate. The prepared kit is used for detecting the antibody and has the effects of high specificity, good repeatability and high sensitivity.
Owner:中山生物工程有限公司
Hepatitis C virus antibody rapid test strips
ActiveCN109212220BHigh compliance rateStrong specificityDisease diagnosisBiological testingHepatitis c viralAntigen
This product is a test strip for the rapid detection of hepatitis C virus antibody saliva. The test strip is composed of a bottom plate and a casing. Colloidal gold-labeled Protein G, nitrocellulose membrane coated with recombinant antigen specifically binding to hepatitis C virus antibody (test line), and a goat anti-human IgG (quality control line). The rapid detection test strip has the advantages of good conservation and strong specificity, can detect infection by hepatitis C virus of all genotypes (types 1-6), and is not interfered by hepatitis B virus and the like.
Owner:BEIJING KAWIN TECH SHARE HLDG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com