Kit for detecting hepatitis C virus antibody, detection method and application thereof
A hepatitis C virus and kit technology, applied in the direction of measurement device, analysis by chemical reaction of materials, instruments, etc., can solve the problems of insensitivity and specificity of chemiluminescence immunodiagnostic reagents, etc., to achieve automatic detection, Wide detection range and improved sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] A test kit for detecting hepatitis C virus antibody, comprising the following components:
[0056] 1) Magnetic microsphere system:
[0057] A solution of magnetic microspheres coated with streptavidin, wherein: the working concentration of the magnetic microspheres: 0.5 mg / ml, and the working concentration of streptavidin: 10 μg / ml.
[0058] HCV antigen solution of labeled biotin, wherein: the working concentration of biotin: 500ng / ml, the working concentration of HCV antigen: 50ng / ml.
[0059] 2) Marker system:
[0060] N-(4-aminobutyl)-N-ethylisoluminol (ABEI) labeled fusion protein antibody solution, wherein: ABEI working concentration: 200ng / ml, fusion protein antibody working concentration: 2000ng / ml.
[0061] HCV fusion antigen solution with Flag-tagged protein, wherein: working concentration of HCV fusion antigen with Flag-tagged protein: 500ng / ml.
[0062] The fusion protein antibody is an antibody to the tag protein in the HCV fusion antigen with tag protein...
experiment example
[0118] Experimental comparisons were carried out using the kit for detecting hepatitis C virus antibody and the detection method thereof in the above-mentioned examples and comparative examples.
[0119] The test samples included: national standard anti-HCV reagents purchased from China National Institute for the Control of Pharmaceutical and Biological Products (batch number: 901), and 114 clinical samples of hepatitis B collected from a hospital in Shenzhen (tested by Abbott anti-HCV ELISA). Using the Maglumi 2000 automatic chemiluminescence analyzer developed and produced by Shenzhen New Industry Biomedical Engineering Co., Ltd., the results are as follows.
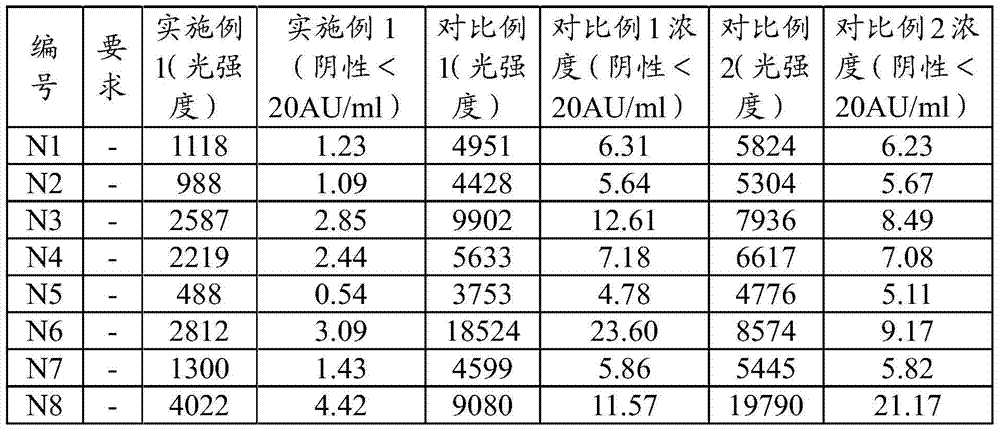
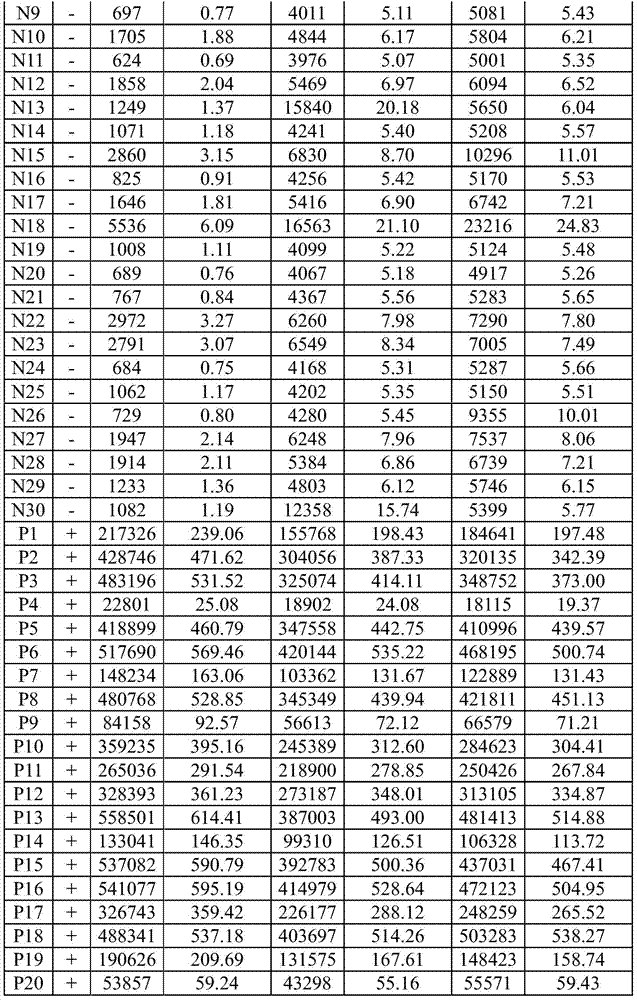
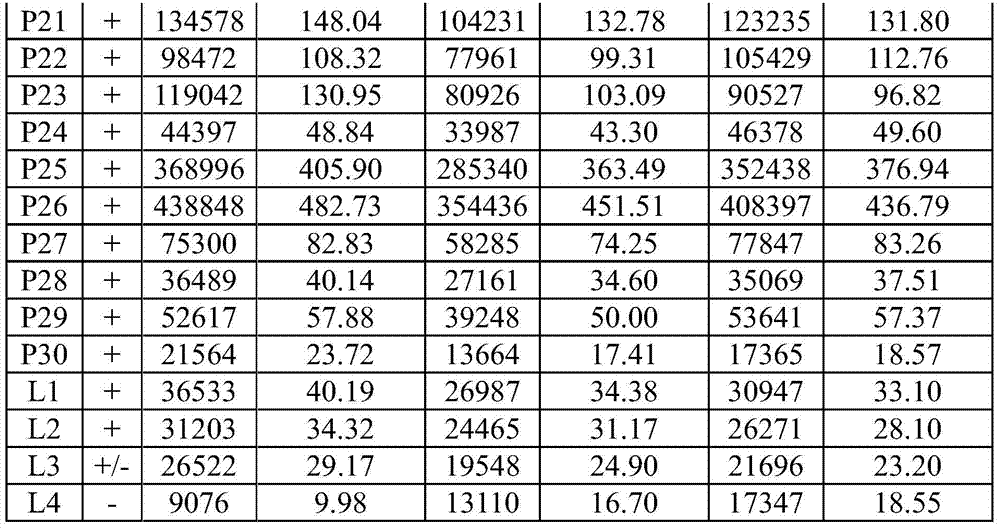
[0120] Table 1. Results of determination of national standard products
[0121]
[0122]
[0123]
[0124] The detection requirements of the national standard products of HCV antibody test reagents are: the coincidence rate of negative national reference products (N1-N30) reaches ≥29 / 30; the coincidence rate o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com